Octyl potassium phosphate
PubChem CID
29394
Molecular Formula
Synonyms
- Dipotassium octyl phosphate
- 19045-79-5
- OCTYL POTASSIUM PHOSPHATE
- Octyl dipotassium phosphate
- Phosphoric acid, octyl ester, potassium salt
Molecular Weight
286.39 g/mol
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Parent Compound
Component Compounds
Dates
- Create:2005-08-08
- Modify:2025-01-04
Chemical Structure Depiction
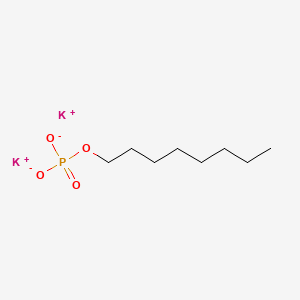
3D Conformer of Parent
dipotassium;octyl phosphate
Computed by Lexichem TK 2.7.0 (PubChem release 2021.10.14)
InChI=1S/C8H19O4P.2K/c1-2-3-4-5-6-7-8-12-13(9,10)11;;/h2-8H2,1H3,(H2,9,10,11);;/q;2*+1/p-2
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
LPZZAIMVFFLHQU-UHFFFAOYSA-L
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
CCCCCCCCOP(=O)([O-])[O-].[K+].[K+]
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C8H17K2O4P
Computed by PubChem 2.2 (PubChem release 2021.10.14)
19045-79-5
51404-72-9
39380-81-9, 97708-79-7
- Dipotassium octyl phosphate
- 19045-79-5
- OCTYL POTASSIUM PHOSPHATE
- Octyl dipotassium phosphate
- Phosphoric acid, octyl ester, potassium salt
- Dipotassium monooctyl phosphate
- UNII-O2J6A53647
- HSDB 2620
- EINECS 242-782-8
- dipotassium;octyl phosphate
- Phosphoric acid, monooctyl ester, potassium salt (1:2)
- DTXSID20889672
- Phosphoric acid, monooctyl ester, dipotassium salt
- O2J6A53647
- 51404-72-9
- SCHEMBL118084
- LPZZAIMVFFLHQU-UHFFFAOYSA-L
- DTXCID401028924
- NS00083067
- DIPOTASSIUM MONOOCTYL PHOSPHATE [HSDB]
- Phosphoric acid, monooctyl ester, potassium salt
- Q27285244
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
286.39 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Donor Count
Property Value
0
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Acceptor Count
Property Value
4
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Rotatable Bond Count
Property Value
7
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Exact Mass
Property Value
286.01385899 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Monoisotopic Mass
Property Value
286.01385899 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Topological Polar Surface Area
Property Value
72.4 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Heavy Atom Count
Property Value
15
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
143
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
3
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
0.00000002 [mmHg]
Other Classes -> Organophosphates, Other
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Parent, Exact Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
Same Count
Sources/Uses
Polyphosphated octyl alcohol: Used as a surface active agent; [HSDB]
SURFACE-ACTIVE AGENT /OCTYL ALCOHOL, POLYPHOSPHATED/
SRI. 1994 Directory of Chemical Producers -United States of America. Menlo Park, CA: SRI International, 1994., p. 105
REACTION OF N-OCTYL ALCOHOL & PHOSPHORUS OXYCHLORIDE, FOLLOWED BY THE REACTION OF THE RESULTING OCTYL PHOSPHATE WITH POTASSIUM HYDROXIDE
SRI
(1972) PROBABLY GREATER THAN 4.54X10+5 GRAMS
SRI
(1974) PROBABLY GREATER THAN 4.54X10+5 GRAMS
SRI
EPA TSCA Commercial Activity Status
Phosphoric acid, monooctyl ester, potassium salt (1:2): ACTIVE
EPA TSCA Commercial Activity Status
Phosphoric acid, octyl ester, potassium salt: ACTIVE
SRP: At the time of review, criteria for land treatment or burial (sanitary landfill) disposal practices are subject to significant revision. Prior to implementing land disposal of waste residue (including waste sludge), consult with environmental regulatory agencies for guidance on acceptable disposal practices.
New Zealand EPA Inventory of Chemical Status
Phosphoric acid, octyl ester, potassium salt: Does not have an individual approval but may be used under an appropriate group standard
Octyl potassium phosphate's production and possible use in the manufacture of octyl polyphosphate, an anionic surface active agent, may result in its release to the environment through various waste streams. If released to the atmosphere, octyl potassium phosphate will exist solely in the particulate phase where it may be removed physically via wet and dry deposition. Octyl potassium phosphate is expected to have very high mobility in soil based on an estimated Koc of 1.3; leaching of octyl potassium phosphate may be possible. Hydrolysis of octyl potassium phosphate may be an important fate process for this compound, particularly under alkaline conditions in either soil or water. Biodegradation of octyl potassium phosphate is possible and may be an important fate process; 57-92% biodegradation of a mono- and dioctyl potassium phosphate mixture was measured via BOD over a 4 week period using an activated sludge inoculum. Volatilization from moist soil surfaces or from water surfaces is unlikely as this compound is a potassium salt. Octyl potassium phosphate will not bioconcentrate in aquatic organisms based on an estimated BCF of 0. (SRC)
Octyl potassium phosphate's production and possible use in the manufacture of octyl polyphosphate(1), an anionic surface active agent, may result in its release to the environment through various waste streams.
(1) SRI International; 1994 Directory of Chemical Producers. USA p 105 (1994)
TERRESTRIAL FATE: An estimated Koc of 1.3(1) indicates that octyl potassium phosphate will have very high mobility in soil(2); leaching of this compound is likely as it should be very soluble in water(SRC). Under alkaline soil conditions, hydrolysis of octyl potassium phosphate may be rapid; the rate of hydrolysis in neutral or slightly acidic soil environments should be slower(3). Biodegradation of octyl potassium phosphate in soil may occur; 57-92% biodegradation of a mixture of the mono- and dioctyl phosphate esters was measured over a 4 week time period using an activated sludge inoculum(4). Octyl potassium phosphate is not expected to volatilize from soil surfaces as it is a salt(SRC).
(1) Lyman WJ et al; Handbook of Chemical Property Estimation Methods. Washington DC: Amer Chem Soc pp. 4-9 (1990)
(2) Swann RL et al; Res Rev 85: 23 (1983)
(3) Muir DCG; in The Handbook of Environmental Chemistry; Anthropogenic Sources. Germany: Springer-Berlag Berlin 3: 41-66 (1984)
(4) Chemicals Inspection and Testing Institute; Japan Chemical Industry Ecology-Toxicology and Information Center. ISBN 4-89074-101-1 (1992)
AQUATIC FATE: Hydrolysis of octyl potassium phosphate, particularly in alkaline waters, may be a major fate process for this compound; in neutral or slightly acidic water, octyl potassium phosphate may hydrolyze less rapidly(1). If released to an aquatic environment, octyl potassium phosphate will not bioconcentrate in aquatic organisms based on an estimated BCF of 0(2,3). Octyl potassium phosphate is not expected to adsorb onto particulate matter or sediment in the water column(3). It is likely that octyl potassium phosphate will biodegrade; a biodegradation study using a mixture of mono- and dioctyl potassium phosphate esters measured via BOD 57-92% biodegradation of these compounds(3). Volatilization of octyl potassium phosphate from water surfaces will not occur as this compound has a high estimated water solubility and low estimated vapor pressure(SRC).
(1) Muir DCG; in The Handbook of Environmental Chemistry; Anthropogenic Sources. Germany: Springer-Berlag Berlin 3: 41-66 (1984)
(2) Lyman WJ et al; Handbook of Chemical Property Estimation Methods Washington, DC: Amer Chem Soc p. 5-4, 4-9 (1990)
ATMOSPHERIC FATE: As a dipotassium salt, octyl potassium phosphate is expected to exist solely as a particulate in the ambient atmosphere. Particulate phase octyl potassium phosphate may be removed physically from the air by wet and dry deposition. (SRC)
57 to 92% biodegradation of a mixture of the mono- and dioctyl potassium phosphate ester was shown via BOD in a 4 week experiment using activated sludge as an inoculum at 30 mg/l and with octyl potassium phosphate present at 100 mg/ml(4).
(1) Chemicals Inspection and Testing Institute; Japan Chemical Industry Ecology-Toxicology and Information Center. ISBN 4-89074-101-1 (1992)
Hydrolysis of octyldiphenyl phosphate may be a major fate process for this compound particularly as octyl potassium phosphate, as a potassium salt, should be very water soluble(SRC). Hydrolysis of phosphate esters is generally promoted by alkaline conditions. The phosphate ester undergoes second order nucleophilic reactions due to the hydroxide ion involving cleavage of the O-P bond; C-O cleavage is dominant for those compounds which undergo acid and water promoted hydrolyses of esters(1). Mono phosphate esters are more likely to hydrolyze than the equivalent di- or triester(1). Octyl potassium phosphate should exist mainly as the anionic form in the environment based on a roughly estimated pKa of 5(2,SRC).
(1) Mabey W, Mill T; J Phys Chem Ref Data 7: 383-415 (1978)
(2) Perrin DD et al; in pKa, Prediction for Organic Acids and Bases Chapman and Hall Ltd, New York, NY p. 91 (1981)
Based on an estimated log Kow of -2.31(1), the BCF of octyl potassium phosphate is estimated as 0 from a regression-derived equation(2,SRC). This BCF value indicates that bioconcentration of this compound in aquatic organisms is not an important fate process(SRC).
(1) Meylan WM, Howard PH; J Pharm Sci 84: 83-92 (1995)
(2) Lyman WJ et al; Handbook of Chemical Property Estimation Methods Washington, DC: Amer Chem Soc p. 5-4 (1990)
Using an estimated log Kow of -2.31(1), the Koc for octyl potassium phosphate was estimated as 1.3(2). According to a suggested classification scheme, this Koc value indicates that octyl potassium phosphate will be highly mobile in soil(3).
(1) Meylan WM, Howard PH; J Pharm Sci 84: 83-92 (1995)
(2) Lyman WJ et al; Handbook of Chemical Property Estimation Methods. Washington DC: Amer Chem Soc pp. 4-9 (1990)
(3) Swann RL et al; Res Rev 85: 23 (1983)
Octyl potassium phosphate is a dipotassium salt which is not expected to volatilize from water surfaces based on its low vapor pressure and high water solubility. (SRC)
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=LPZZAIMVFFLHQU-UHFFFAOYSA-L
- ChemIDplusOctyl potassium phosphatehttps://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0019045795Phosphoric acid, octyl ester, potassium salthttps://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0051404729ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA Chemicals under the TSCAPhosphoric acid, monooctyl ester, potassium salt (1:2)https://www.epa.gov/chemicals-under-tscaEPA TSCA Classificationhttps://www.epa.gov/tsca-inventory
- EPA DSSToxPhosphoric acid, monooctyl ester, potassium salt (1:2)https://comptox.epa.gov/dashboard/DTXSID20889672CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeDipotassium octyl phosphatehttps://echa.europa.eu/substance-information/-/substanceinfo/100.038.878
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingDIPOTASSIUM OCTYL PHOSPHATEhttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/O2J6A53647
- Hazardous Substances Data Bank (HSDB)OCTYL POTASSIUM PHOSPHATEhttps://pubchem.ncbi.nlm.nih.gov/source/hsdb/2620
- New Zealand Environmental Protection Authority (EPA)LICENSEThis work is licensed under the Creative Commons Attribution-ShareAlike 4.0 International licence.https://www.epa.govt.nz/about-this-site/general-copyright-statement/Phosphoric acid, octyl ester, potassium salthttps://www.epa.govt.nz/industry-areas/hazardous-substances/guidance-for-importers-and-manufacturers/hazardous-substances-databases/
- Haz-Map, Information on Hazardous Chemicals and Occupational DiseasesLICENSECopyright (c) 2022 Haz-Map(R). All rights reserved. Unless otherwise indicated, all materials from Haz-Map are copyrighted by Haz-Map(R). No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://haz-map.com/AboutOctyl potassium phosphatehttps://haz-map.com/Agents/6440
- Japan Chemical Substance Dictionary (Nikkaji)
- Wikidatadipotassium octyl phosphatehttps://www.wikidata.org/wiki/Q27285244
- PubChem
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 388485409https://pubchem.ncbi.nlm.nih.gov/substance/388485409
CONTENTS
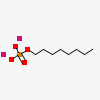

 CID 19892 (Monooctyl phosphate)
CID 19892 (Monooctyl phosphate) CID 5462222 (Potassium)
CID 5462222 (Potassium)