1-O-Octadecyl-SN-glycero-3-phosphocholine
PubChem CID
2733532
Molecular Formula
Synonyms
- 1-O-OCTADECYL-SN-GLYCERO-3-PHOSPHOCHOLINE
- 74430-89-0
- 1-Octadecyl-sn-glycero-3-phosphocholine
- PC(O-18:0/0:0)
- LysoPC(O-18:0)
Molecular Weight
509.7 g/mol
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Parent Compound
Dates
- Create:2005-07-19
- Modify:2025-01-18
Description
1-O-octadecyl-sn-glycero-3-phosphocholine is a 1-alkyl-sn-glycero-3-phosphocholine in which the alkyl group is specified as octadecyl It is an organic molecular entity and a 1-alkyl-sn-glycero-3-phosphocholine.
1-O-Octadecyl-SN-glycero-3-phosphocholine has been reported in Marphysa sanguinea with data available.
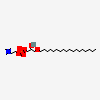
Chemical Structure Depiction

Conformer generation is disallowed since too flexible
[(2R)-2-hydroxy-3-octadecoxypropyl] 2-(trimethylazaniumyl)ethyl phosphate
Computed by Lexichem TK 2.7.0 (PubChem release 2024.11.20)
InChI=1S/C26H56NO6P/c1-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-22-31-24-26(28)25-33-34(29,30)32-23-21-27(2,3)4/h26,28H,5-25H2,1-4H3/t26-/m1/s1
Computed by InChI 1.07.0 (PubChem release 2024.11.20)
XKBJVQHMEXMFDZ-AREMUKBSSA-N
Computed by InChI 1.07.0 (PubChem release 2024.11.20)
CCCCCCCCCCCCCCCCCCOC[C@H](COP(=O)([O-])OCC[N+](C)(C)C)O
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C26H56NO6P
Computed by PubChem 2.2 (PubChem release 2024.11.20)
- 1-O-OCTADECYL-SN-GLYCERO-3-PHOSPHOCHOLINE
- 74430-89-0
- 1-Octadecyl-sn-glycero-3-phosphocholine
- PC(O-18:0/0:0)
- LysoPC(O-18:0)
- [(2R)-2-hydroxy-3-octadecoxypropyl] 2-(trimethylazaniumyl)ethyl phosphate
- 1-O-Octadecylglycerol-3-phosphatidylcholine
- LysoPC(18:0e/0:0)
- CHEBI:75216
- 1-o-octadecyl-2-hydroxy-sn-glycero-3-phosphocholine
- Ro 14-8160
- LPC(o18:0)
- 1-stearyl-GPC
- LysoPC(dm18:0)
- LYSO-PAF C18
- BML3-E06
- CHEMBL475587
- SCHEMBL6847801
- 1-stearyl-GPC (O-18:0)
- DTXSID30274414
- LysoPC(O-18:0/0:0)
- XKBJVQHMEXMFDZ-AREMUKBSSA-N
- HMS3648P20
- GPC(O-18:0)
- LMGP01060014
- MFCD00065889
- GPC(O-18:0/0:0)
- NCGC00161376-01
- HY-141581
- CS-0181734
- NS00074218
- 1-O-Octadecyl-sn-glyceryl-3-phosphorylcholine
- G91122
- 1- O- octadecyl- sn- glyceryl- 3- phosphorylcholine
- Q27105036
- (R)-2-Hydroxy-3-(octadecyloxy)propyl (2-(trimethylammonio)ethyl) phosphate
- (R)-2-hydroxy-3-(octadecyloxy)propyl 2-(trimethylammonio)ethyl phosphate
- C18 Lyso PAF, 1-O-octadecyl-2-hydroxy-sn-glycero-3-phosphocholine, powder
- C18 Lyso PAF, 1-O-octadecyl-2-hydroxy-sn-glycero-3-phosphocholine, chloroform
- 3,5,9-Trioxa-4-phosphaheptacosan-1-aminium, 4,7-dihydroxy-N,N,N-trimethyl-, inner salt, 4-oxide, (R)-
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
509.7 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Property Name
XLogP3-AA
Property Value
7
Reference
Computed by XLogP3 3.0 (PubChem release 2024.11.20)
Property Name
Hydrogen Bond Donor Count
Property Value
1
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Hydrogen Bond Acceptor Count
Property Value
6
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Rotatable Bond Count
Property Value
26
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Exact Mass
Property Value
509.38452550 Da
Reference
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Property Name
Monoisotopic Mass
Property Value
509.38452550 Da
Reference
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Property Name
Topological Polar Surface Area
Property Value
88.1 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Heavy Atom Count
Property Value
34
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
478
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
1
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2019.01.04)
Solid
238.2 Ų [M+H]+ [CCS Type: TIMS; Method: calibrated with 4 ions from ESI LC/MS tuning mix (Agilent)]
238.4 Ų [M+H]+ [CCS Type: TIMS; Method: calibrated with 4 ions from ESI LC/MS tuning mix (Agilent)]
240.1 Ų [M+CH3COO]- [CCS Type: TIMS; Method: single field calibrated]
Lipids -> Glycerophospholipids [GP] -> Glycerophosphocholines [GP01] -> Monoalkylglycerophosphocholines [GP0106]
NIST Number
1118012
Instrument Type
IT/ion trap
Collision Energy
0
Spectrum Type
MS2
Precursor Type
[M+H]+
Precursor m/z
510.3918
Total Peaks
11
m/z Top Peak
184.1
m/z 2nd Highest
492.4
m/z 3rd Highest
433.2
Thumbnail
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Stereo Count
Same Isotope Count
Same Parent, Connectivity Count
Same Parent, Stereo Count
Same Parent, Isotope Count
Same Parent, Exact Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
Same Count
- Extracellular
- Membrane
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=XKBJVQHMEXMFDZ-AREMUKBSSA-N
The LOTUS Initiative for Open Natural Products Research: frozen dataset union wikidata (with metadata) | DOI:10.5281/zenodo.5794106
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/1-O-Octadecyl-sn-glycero-3-phosphocholinehttps://commonchemistry.cas.org/detail?cas_rn=74430-89-0
- EPA DSSTox1-O-Octadecyl-sn-glycero-3-phosphocholinehttps://comptox.epa.gov/dashboard/DTXSID30274414CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citingLysoPC(O-18:0/0:0)http://www.hmdb.ca/metabolites/HMDB0011149
- CCSbaseCCSbase Classificationhttps://ccsbase.net/
- ChEBI1-O-octadecyl-sn-glycero-3-phosphocholinehttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:75216
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/1-O-Octadecyl-SN-glycero-3-phosphocholinehttps://www.wikidata.org/wiki/Q27105036LOTUS Treehttps://lotus.naturalproducts.net/
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.html
- FooDBLICENSEFooDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (FooDB) and the original publication.https://foodb.ca/aboutLysoPC(O-18:0)https://foodb.ca/compounds/FDB027924
- LIPID MAPSLipid Classificationhttps://www.lipidmaps.org/
- Metabolomics Workbench
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-lawLyso-PAF C-18http://www.nist.gov/srd/nist1a.cfm
- Rhea - Annotated Reactions DatabaseLICENSERhea has chosen to apply the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/). This means that you are free to copy, distribute, display and make commercial use of the database in all legislations, provided you credit (cite) Rhea.https://www.rhea-db.org/help/license-disclaimer
- Wikidata1-O-octadecyl-sn-glycero-3-phosphocholinehttps://www.wikidata.org/wiki/Q27105036
- PubChem
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 388546193https://pubchem.ncbi.nlm.nih.gov/substance/388546193
CONTENTS
 CID 446876 (Lpc-Ether)
CID 446876 (Lpc-Ether)