N-(5-Guanidino-1-((4-nitrophenyl)amino)-1-oxopentan-2-yl)benzamide hydrochloride
PubChem CID
2724371
Molecular Formula
Synonyms
- 911-77-3
- BAPNA
- N-(5-Guanidino-1-((4-nitrophenyl)amino)-1-oxopentan-2-yl)benzamide hydrochloride
- N-Benzoyl-DL-arginine-4-nitroanilide hydrochloride
- Bz-DL-Arg-pNA HCl
Molecular Weight
434.9 g/mol
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Parent Compound
Component Compounds
Dates
- Create:2005-07-19
- Modify:2025-01-18
Description
A chromogenic substrate that permits direct measurement of peptide hydrolase activity, e.g., papain and trypsin, by colorimetry. The substrate liberates p-nitroaniline as a chromogenic product.
Chemical Structure Depiction
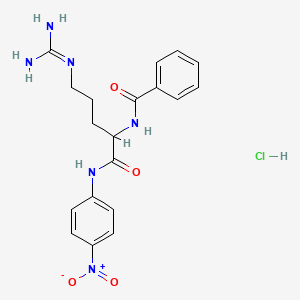
3D Conformer of Parent
SVG Image

IUPAC Condensed
Bz-DL-Arg-pNA.HCl
Sequence
R
N-[5-(diaminomethylideneamino)-1-(4-nitroanilino)-1-oxopentan-2-yl]benzamide;hydrochloride
Computed by Lexichem TK 2.7.0 (PubChem release 2021.10.14)
InChI=1S/C19H22N6O4.ClH/c20-19(21)22-12-4-7-16(24-17(26)13-5-2-1-3-6-13)18(27)23-14-8-10-15(11-9-14)25(28)29;/h1-3,5-6,8-11,16H,4,7,12H2,(H,23,27)(H,24,26)(H4,20,21,22);1H
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
DEOKFPFLXFNAON-UHFFFAOYSA-N
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
C1=CC=C(C=C1)C(=O)NC(CCCN=C(N)N)C(=O)NC2=CC=C(C=C2)[N+](=O)[O-].Cl
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C19H23ClN6O4
Computed by PubChem 2.2 (PubChem release 2021.10.14)
37039-24-0, 99671-52-0
- BAPNA
- Benzoylarginine Nitroanilide
- Benzoylarginine Nitroanilide Monohydrochloride
- Benzoylarginine Nitroanilide, (R)-Isomer
- Benzoylarginine Nitroanilide, (S)-Isomer
- Benzoylarginine Nitroanilide, Monosodium Salt, Monohydrochloride
- Monohydrochloride, Benzoylarginine Nitroanilide
- N alpha Benzoyl DL arginine 4 nitroanilide
- N Benzoylarginine 4 nitroanilide
- N Benzoylarginyl 4 nitroanilide
- N-alpha-Benzoyl-DL-arginine-4-nitroanilide
- N-Benzoylarginine-4-nitroanilide
- N-Benzoylarginyl-4-nitroanilide
- Nitroanilide, Benzoylarginine
- 911-77-3
- BAPNA
- N-(5-Guanidino-1-((4-nitrophenyl)amino)-1-oxopentan-2-yl)benzamide hydrochloride
- N-Benzoyl-DL-arginine-4-nitroanilide hydrochloride
- Bz-DL-Arg-pNA HCl
- DL-BAPA . HCl;DL-BApNA . HCl
- Benzamide,N-[4-[(aminoiminomethyl)amino]-1-[[(4-nitrophenyl)amino]carbonyl]butyl]-, monohydrochloride
- Nalpha-Benzoyl-DL-arginine 4-nitroanilide hydrochloride
- N-Benzoyl-dl-arginine-4-nitroanilide HCl
- 5-carbamimidamido-N-(4-nitrophenyl)-2-(phenylformamido)pentanamide hydrochloride
- N-[5-(diaminomethylideneamino)-1-(4-nitroanilino)-1-oxopentan-2-yl]benzamide;hydrochloride
- Bz-Arg-pNA HCl
- L-BApNA . HCl;L-BAPA . HCl
- D-BAPA . HCl;D-BApNA . HCl
- MFCD00012846
- N-alpha-Benzoyl-DL-arginine-4-nitroanilide hydrochloride
- Bz-D-Arg-pNA.HCl
- Bz-DL-Arg-PNA.HCl
- C19H23ClN6O4
- MLS001361402
- SCHEMBL2044745
- CHEMBL1898160
- DTXSID50883603
- DL-BAPA HCl;DL-BApNA HCl
- AKOS015902372
- CS-W009669
- AS-74759
- PD150662
- SMR000875358
- DB-057243
- B-0800
- D81794
- n-benzoyl-dl-arginine p-nitroanilide hydrochloride
- Na-Benzoyl-L-arginine 4-nitroanilide hydrochloride
- Na-Benzoyl-DL-arginine-4-nitroanilide hydrochloride
- N-|A-Benzoyl-DL-arginine 4-nitroanilide hydrochloride
- Nalpha-Benzoyl-DL-arginine p-nitroanilide hydrochloride
- N-alpha-Benzoyl-DL-arginine 4-nitroanilide monohydrochloride
- N- alpha -Benzoyl-DL-arginine 4-nitroanilide monohydrochloride
- Nalpha-Benzoyl-DL-arginine 4-nitroanilide hydrochloride, >=98%
- N-(5-guanidino-1-(4-nitrophenylamino)-1-oxopentan-2-yl)benzamide hydrochloride
- N-[(2S)-5-(diaminomethylideneamino)-1-(4-nitroanilino)-1-oxopentan-2-yl]benzamide,hydrochloride
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
434.9 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Donor Count
Property Value
5
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Acceptor Count
Property Value
5
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Rotatable Bond Count
Property Value
8
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Exact Mass
Property Value
434.1469309 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Monoisotopic Mass
Property Value
434.1469309 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Topological Polar Surface Area
Property Value
168 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Heavy Atom Count
Property Value
30
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
588
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
1
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
2
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
Instrument Name
Bio-Rad FTS
Technique
ATR-Neat
Source of Spectrum
Forensic Spectral Research
Source of Sample
Sigma-Aldrich Company LLC
Catalog Number
<a href=https://www.sigmaaldrich.com/US/en/product/sigma/B4875>B4875</a>
Lot Number
110H0329
Copyright
Copyright © 2019-2024 John Wiley & Sons, Inc. All Rights Reserved.
Technique
FT-Raman
Source of Spectrum
Forensic Spectral Research
Source of Sample
Sigma-Aldrich Company Llc.
Catalog Number
<a href=https://www.sigmaaldrich.com/US/en/product/aldrich/857114>857114</a>
Lot Number
03717JP
Copyright
Copyright © 2013-2024 John Wiley & Sons, Inc. All Rights Reserved.
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Parent, Connectivity Count
Same Parent, Isotope Count
Same Parent, Exact Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
Same Count
Chromogenic Compounds
Colorless, endogenous or exogenous pigment precursors that may be transformed by biological mechanisms into colored compounds; used in biochemical assays and in diagnosis as indicators, especially in the form of enzyme substrates. Synonym: chromogens (not to be confused with pigment-synthesizing bacteria also called chromogens). (See all compounds classified as Chromogenic Compounds.)
EPA TSCA Commercial Activity Status
Benzamide, N-[4-[(aminoiminomethyl)amino]-1-[[(4-nitrophenyl)amino]carbonyl]butyl]-, hydrochloride (1:1): INACTIVE
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=DEOKFPFLXFNAON-UHFFFAOYSA-N
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/N-α-Benzoyl-DL-arginine-p-nitroanilide hydrochloridehttps://commonchemistry.cas.org/detail?cas_rn=911-77-3
- EPA Chemicals under the TSCABenzamide, N-[4-[(aminoiminomethyl)amino]-1-[[(4-nitrophenyl)amino]carbonyl]butyl]-, hydrochloride (1:1)https://www.epa.gov/chemicals-under-tscaEPA TSCA Classificationhttps://www.epa.gov/tsca-inventory
- EPA DSSToxBenzamide, N-[4-[(aminoiminomethyl)amino]-1-[[(4-nitrophenyl)amino]carbonyl]butyl]-, hydrochloride (1:1)https://comptox.epa.gov/dashboard/DTXSID50883603CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.html
- SpectraBaseN alpha-Benzoyl-DL-arginine-4-nitroanilide HClhttps://spectrabase.com/spectrum/AJ1RR0NWeD4Nα-Benzoyl-DL-arginine p-nitroanilide hydrochloridehttps://spectrabase.com/spectrum/4Z4QXWX4FQCDL-N-{4-GUANIDINO-1-[(p-NITROPHENYL)CARBAMOYL]BUTYL}BENZAMIDE,MONOHYDROCHLORIDEhttps://spectrabase.com/spectrum/8yrPOlkEv8KL-N-{4-GUANIDINO-1-[(p-NITROPHENYL)CARBAMOYL]BUTYL}BENZAMIDE,MONOHYDROCHLORIDEhttps://spectrabase.com/spectrum/8GPA9w3iI9oN alpha-Benzoyl-DL-arginine-4-nitroanilide HClhttps://spectrabase.com/spectrum/BQykRRGUhwMNα-Benzoyl-DL-arginine p-nitroanilide hydrochloridehttps://spectrabase.com/spectrum/IiwrOLJVBtP
- WikidataN-Benzoyl-DL-arginine-4-nitroanilide hydrochloridehttps://www.wikidata.org/wiki/Q72440200
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlBenzoylarginine Nitroanilidehttps://www.ncbi.nlm.nih.gov/mesh/68001586Chromogenic Compoundshttps://www.ncbi.nlm.nih.gov/mesh/68002863
- PubChem
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 397702275https://pubchem.ncbi.nlm.nih.gov/substance/397702275
CONTENTS
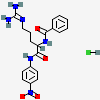

 CID 13494 (Benzoylarginine nitroanilide)
CID 13494 (Benzoylarginine nitroanilide) CID 313 (Hydrochloric Acid)
CID 313 (Hydrochloric Acid)