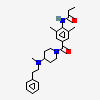
OPC-28326 free base
PubChem CID
219020
Molecular Formula
Synonyms
- OPC-28326
- 167626-17-7
- OPC-28326 free base
- 77QB6Y63UM
- UNII-77QB6Y63UM
Molecular Weight
421.6 g/mol
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Dates
- Create:2005-08-09
- Modify:2025-01-11
Description
At low doses, OPC 28326 selectively vasodilates the femoral arterial bed due to its inhibitory action at alpha-2-adrenoceptors while having minimal action on systemic blood pressure, heart rate and coronary, carotid, vertebral, renal, and mesenteric blood flows. It is the only clinical compound with this profile. It is currently being investigated in the treatment of peripheral vascular diseases and Raynaud's syndrome.
Chemical Structure Depiction
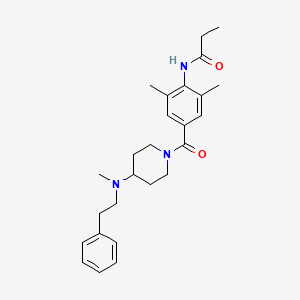
N-[2,6-dimethyl-4-[4-[methyl(2-phenylethyl)amino]piperidine-1-carbonyl]phenyl]propanamide
Computed by Lexichem TK 2.7.0 (PubChem release 2024.11.20)
InChI=1S/C26H35N3O2/c1-5-24(30)27-25-19(2)17-22(18-20(25)3)26(31)29-15-12-23(13-16-29)28(4)14-11-21-9-7-6-8-10-21/h6-10,17-18,23H,5,11-16H2,1-4H3,(H,27,30)
Computed by InChI 1.07.0 (PubChem release 2024.11.20)
BESKMDLUOAVUJF-UHFFFAOYSA-N
Computed by InChI 1.07.0 (PubChem release 2024.11.20)
CCC(=O)NC1=C(C=C(C=C1C)C(=O)N2CCC(CC2)N(C)CCC3=CC=CC=C3)C
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C26H35N3O2
Computed by PubChem 2.2 (PubChem release 2024.11.20)
167626-17-7
- 4-(N-methyl-2-phenylethylamino)-1-(3,5-dimethyl-4-propionylaminobenzoyl)piperidine
- OPC 28326
- OPC-28326
- OPC-28326
- 167626-17-7
- OPC-28326 free base
- 77QB6Y63UM
- UNII-77QB6Y63UM
- N-[2,6-dimethyl-4-[4-[methyl(2-phenylethyl)amino]piperidine-1-carbonyl]phenyl]propanamide
- 4-(N-Methyl-2-phenylethylamino)-1-(3, 5-dimethyl-4-propionylaminobenzoyl)piperidine
- Propanamide, N-(2,6-dimethyl-4-((4-(methyl(2-phenylethyl)amino)-1-piperidinyl)carbonyl)phenyl)-
- SCHEMBL2831451
- CHEMBL4742102
- BDBM85825
- PDSP1_000826
- PDSP2_000813
- AKOS040740863
- CAS_219019
- CS-6725
- NSC_219019
- DA-66353
- TS-07942
- HY-101610
- NS00069717
- L001587
- N-(2,6-Dimethyl-4-(4-(methyl(phenethyl)amino)piperidine-1-carbonyl)phenyl)propionamide
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
421.6 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Property Name
XLogP3-AA
Property Value
4.3
Reference
Computed by XLogP3 3.0 (PubChem release 2024.11.20)
Property Name
Hydrogen Bond Donor Count
Property Value
1
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Hydrogen Bond Acceptor Count
Property Value
3
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Rotatable Bond Count
Property Value
7
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Exact Mass
Property Value
421.27292737 Da
Reference
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Property Name
Monoisotopic Mass
Property Value
421.27292737 Da
Reference
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Property Name
Topological Polar Surface Area
Property Value
52.7 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Heavy Atom Count
Property Value
31
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
569
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
Pharmaceuticals -> Listed in ZINC15
S55 | ZINC15PHARMA | Pharmaceuticals from ZINC15 | DOI:10.5281/zenodo.3247749
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Parent, Exact Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
PubMed Count
Investigated for use/treatment in peripheral vascular disease and raynaud's disease.
At low doses, OPC 28326 selectively vasodilates the femoral arterial bed due to its inhibitory action at alpha-2-adrenoceptors while having minimal action on systemic blood pressure, heart rate and coronary, carotid, vertebral, renal, and mesenteric blood flows.
At low doses, OPC 28326 selectively vasodilates the femoral arterial bed due to its inhibitory action at alpha-2C-adrenoceptors. Other studies have also reported selectivity for a-2B-adrenoceptors.
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=BESKMDLUOAVUJF-UHFFFAOYSA-N
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.html
- ChemIDplusOPC-28326 free basehttps://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0167626177ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingOPC-28326 FREE BASEhttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/77QB6Y63UM
- DrugBankLICENSECreative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode)https://www.drugbank.ca/legal/terms_of_use
- Therapeutic Target Database (TTD)
- Japan Chemical Substance Dictionary (Nikkaji)
- Metabolomics Workbench
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/Opc-28326NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- WikidataN-[2,6-dimethyl-4-[4-[methyl(2-phenylethyl)amino]piperidine-1-carbonyl]phenyl]propanamidehttps://www.wikidata.org/wiki/Q126601013
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.html
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 390507396https://pubchem.ncbi.nlm.nih.gov/substance/390507396
CONTENTS