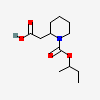
2-(1-sec-Butoxycarbonyl-2-piperidyl)acetic acid
PubChem CID
85992116
Molecular Formula
Synonyms
- SCHEMBL15271618
- OMRINPAHALFCIC-UHFFFAOYSA-N
- DS-022150
- 2-(1-sec-Butoxycarbonyl-2-piperidyl)acetic acid
- 749864-16-2
Molecular Weight
243.30 g/mol
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Dates
- Create:2014-11-03
- Modify:2025-01-18
Chemical Structure Depiction
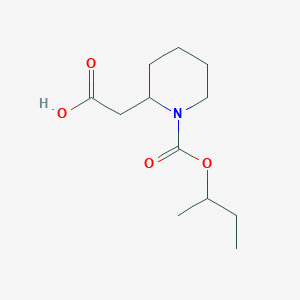
2-(1-butan-2-yloxycarbonylpiperidin-2-yl)acetic acid
Computed by LexiChem 2.6.6 (PubChem release 2019.06.18)
InChI=1S/C12H21NO4/c1-3-9(2)17-12(16)13-7-5-4-6-10(13)8-11(14)15/h9-10H,3-8H2,1-2H3,(H,14,15)
Computed by InChI 1.0.5 (PubChem release 2019.06.18)
OMRINPAHALFCIC-UHFFFAOYSA-N
Computed by InChI 1.0.5 (PubChem release 2019.06.18)
CCC(C)OC(=O)N1CCCCC1CC(=O)O
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C12H21NO4
Computed by PubChem 2.1 (PubChem release 2019.06.18)
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
243.30 g/mol
Reference
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Property Name
XLogP3-AA
Property Value
1.9
Reference
Computed by XLogP3 3.0 (PubChem release 2019.06.18)
Property Name
Hydrogen Bond Donor Count
Property Value
1
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Hydrogen Bond Acceptor Count
Property Value
4
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Rotatable Bond Count
Property Value
5
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Exact Mass
Property Value
243.14705815 Da
Reference
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Property Name
Monoisotopic Mass
Property Value
243.14705815 Da
Reference
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Property Name
Topological Polar Surface Area
Property Value
66.8 Ų
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Heavy Atom Count
Property Value
17
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
280
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
2
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2012.11.26)
Accession ID
Authors
Kevin S. Jewell; Björn Ehlig; Arne Wick
Instrument
TripleTOF 6600 SCIEX
Instrument Type
LC-ESI-QTOF
MS Level
MS2
Ionization Mode
POSITIVE
Ionization
ESI
Collision Energy
10
Fragmentation Mode
CID
Column Name
Zorbax Eclipse Plus C18 2.1 mm x 150 mm, 3.5 um, Agilent
Retention Time
9.741 min
Precursor m/z
244.1543
Precursor Adduct
[M+H]+
Top 5 Peaks
144.1038 999
188.0915 442
170.0796 80
84.0783 43
128.0678 14
License
dl-de/by-2-0
Accession ID
Authors
Kevin S. Jewell; Björn Ehlig; Arne Wick
Instrument
TripleTOF 6600 SCIEX
Instrument Type
LC-ESI-QTOF
MS Level
MS2
Ionization Mode
POSITIVE
Ionization
ESI
Collision Energy
20
Fragmentation Mode
CID
Column Name
Zorbax Eclipse Plus C18 2.1 mm x 150 mm, 3.5 um, Agilent
Retention Time
9.741 min
Precursor m/z
244.1543
Precursor Adduct
[M+H]+
Top 5 Peaks
144.1045 999
84.0791 375
128.0684 86
170.0794 30
188.0904 12
License
dl-de/by-2-0
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=OMRINPAHALFCIC-UHFFFAOYSA-N
CONTENTS