1-O-alpha-D-Glucopyranosyl-d-mannitol dihydrate
PubChem CID
18454344
Molecular Formula
Synonyms
- 1-O-alpha-D-Glucopyranosyl-d-mannitol dihydrate
- 174060-42-5
- 1,1-GPM dihydrate
- UNII-ZY0230UH0D
- ZY0230UH0D
Molecular Weight
380.34 g/mol
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Parent Compound
Component Compounds
Dates
- Create:2007-12-04
- Modify:2024-12-07
Description
1-O-alpha-D-glucopyranosyl-D-mannitol dihydrate is a hydrate that is the dihydrate form of 1-O-alpha-D-glucopyranosyl-D-mannitol. It contains a 1-O-alpha-D-glucopyranosyl-D-mannitol.
Chemical Structure Depiction
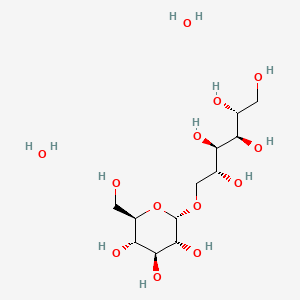
3D Conformer of Parent
(2R,3R,4R,5R)-6-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyhexane-1,2,3,4,5-pentol;dihydrate
Computed by Lexichem TK 2.7.0 (PubChem release 2021.10.14)
InChI=1S/C12H24O11.2H2O/c13-1-4(15)7(17)8(18)5(16)3-22-12-11(21)10(20)9(19)6(2-14)23-12;;/h4-21H,1-3H2;2*1H2/t4-,5-,6-,7-,8-,9-,10+,11-,12+;;/m1../s1
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
BTKQLFSKIFGYOF-MASOBFGXSA-N
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
C([C@@H]1[C@H]([C@@H]([C@H]([C@H](O1)OC[C@H]([C@H]([C@@H]([C@@H](CO)O)O)O)O)O)O)O)O.O.O
Computed by OEChem 2.3.0 (PubChem release 2021.10.14)
C12H28O13
Computed by PubChem 2.2 (PubChem release 2021.10.14)
174060-42-5
- 1-O-alpha-D-Glucopyranosyl-d-mannitol dihydrate
- 174060-42-5
- 1,1-GPM dihydrate
- UNII-ZY0230UH0D
- ZY0230UH0D
- GLUCOSYLMANNITOL DIHYDRATE
- CHEBI:192267
- DTXSID10169753
- GLUCOSYLMANNITOL DIHYDRATE [MI]
- 1-O-(a-Glucopyranosyl)-D-mannitol - Dihydrate
- 1-O-alpha-D-glucopyranosyl-D-mannitol--water (1/2)
- 1-O-.ALPHA.-D-GLUCOPYRANOSYL-D-MANNITOL DIHYDRATE
- 1-O-(a-Glucopyranosyl)-D-mannitol dihydrate
- MFCD00270420
- SCHEMBL901806
- D-Mannitol, 1-O-alpha-D-glucopyranosyl-, dihydrate
- DTXCID5092244
- D-Mannitol, 1-O-alpha-D-glucopyranosyl-, hydrate (1:2)
- D-Mannitol,1-O-a-D-glucopyranosyl-
- 1-O-alpha-D-Glucopyranosyl-D-mannitol dihydrate, 98%
- Q27295938
- D-MANNITOL, 1-O-.ALPHA.-D-GLUCOPYRANOSYL-, DIHYDRATE
- D-MANNITOL, 1-O-.ALPHA.-D-GLUCOPYRANOSYL-, HYDRATE (1:2)
- (2R,3R,4R,5R)-6-(((2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)hexane-1,2,3,4,5-pentaol dihydrate
- (2R,3R,4R,5R)-6-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyhexane-1,2,3,4,5-pentol;dihydrate
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
380.34 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Donor Count
Property Value
11
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Acceptor Count
Property Value
13
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Rotatable Bond Count
Property Value
8
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Exact Mass
Property Value
380.15299094 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Monoisotopic Mass
Property Value
380.15299094 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Topological Polar Surface Area
Property Value
203Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Heavy Atom Count
Property Value
25
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
343
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
9
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
3
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Parent, Connectivity Count
Same Parent, Exact Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
Same Count
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=BTKQLFSKIFGYOF-MASOBFGXSA-N
- ChEBI1-O-alpha-D-glucopyranosyl-D-mannitol dihydratehttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:192267
- ChemIDplus1-O-alpha-D-Glucopyranosyl-d-mannitol dihydratehttps://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0174060425ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSTox1-O-alpha-D-Glucopyranosyl-d-mannitol dihydratehttps://comptox.epa.gov/dashboard/DTXSID10169753CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingGLUCOSYLMANNITOL DIHYDRATEhttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/ZY0230UH0D
- Springer Nature
- Wikidata1-O-α-D-glucopyranosyl-D-mannitol dihydratehttps://www.wikidata.org/wiki/Q27295938
- PubChem
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 389389486https://pubchem.ncbi.nlm.nih.gov/substance/389389486
CONTENTS
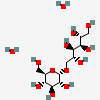

 CID 88735 (1-O-alpha-D-Glucopyranosyl-D-mannitol)
CID 88735 (1-O-alpha-D-Glucopyranosyl-D-mannitol) CID 962 (Water)
CID 962 (Water)