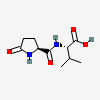
Pyroglutamylvaline
- Pyroglutamylvaline
- 21282-10-0
- PYR-VAL-OH
- Pyro-glu-val
- Pyroglutamyl valine
- Create:2005-08-08
- Modify:2025-01-18
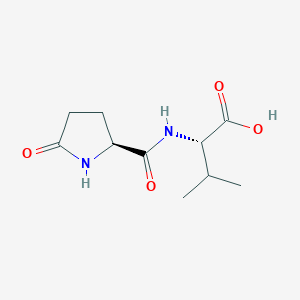

- pyro-Glu-Val
- pyroglutamyl valine
- pyroglutamylvaline
- Pyroglutamylvaline
- 21282-10-0
- PYR-VAL-OH
- Pyro-glu-val
- Pyroglutamyl valine
- (2S)-3-methyl-2-[[(2S)-5-oxopyrrolidine-2-carbonyl]amino]butanoic acid
- p-Glu-Val
- 5-oxo-L-prolyl-L-valine
- CHEBI:132991
- DTXSID40175549
- L-Valine, N-(5-oxo-L-prolyl)-
- ((S)-5-oxopyrrolidine-2-carbonyl)-L-valine
- (2S)-2-[[(2S)-5-ketoprolyl]amino]-3-methyl-butyric acid
- E(Pyro_glu)V
- (2S)-3-METHYL-2-{[(2S)-5-OXOPYRROLIDIN-2-YL]FORMAMIDO}BUTANOIC ACID
- L-pyroglutamyl-L-valine
- (2S)-2-(((2S)-5-ketoprolyl)amino)-3-methyl-butyric acid
- MFCD00037885
- Pyr-Val-OH, AldrichCPR
- SCHEMBL2597779
- DTXCID1098040
- AKOS025627798
- CS-0134004
- EN300-1270311
- Z1455251301
Silke Matysik, Caroline Ivanne Le Roy, Gerhard Liebisch, Sandrine Paule Claus. Metabolomics of fecal samples: A practical consideration. Trends in Food Science & Technology. Vol. 57, Part B, Nov. 2016, p.244-255: http://www.sciencedirect.com/science/article/pii/S0924224416301984
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=DTSWLLBBGHRXQH-XPUUQOCRSA-N
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/5-Oxo-L-prolyl-L-valinehttps://commonchemistry.cas.org/detail?cas_rn=21282-10-0
- ChemIDplusChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSToxPyroglutamylvalinehttps://comptox.epa.gov/dashboard/DTXSID40175549CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citingPyroglutamylvalinehttp://www.hmdb.ca/metabolites/HMDB0094651
- ChEBIPyroglutamylvalinehttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:132991
- Japan Chemical Substance Dictionary (Nikkaji)
- Metabolomics WorkbenchPyroglutamylvalinehttps://www.metabolomicsworkbench.org/data/StructureData.php?RegNo=71627
- Springer Nature
- WikidataPyroglutamylvalinehttps://www.wikidata.org/wiki/Q83045914
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlpyroglutamylvalinehttps://www.ncbi.nlm.nih.gov/mesh/67029730
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 393354263https://pubchem.ncbi.nlm.nih.gov/substance/393354263