Odoratin
PubChem CID
13965473
Molecular Formula
Synonyms
- Odoratin
- 53948-00-8
- 7-hydroxy-3-(3-hydroxy-4-methoxyphenyl)-6-methoxychromen-4-one
- CHEMBL469824
- SCHEMBL1248967
Molecular Weight
314.29 g/mol
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Dates
- Create:2007-02-08
- Modify:2025-01-18
Description
Odoratin is an isoflavonoid.
Odoratin has been reported in Bowdichia virgilioides, Dalbergia louvelii, and other organisms with data available.
Chemical Structure Depiction
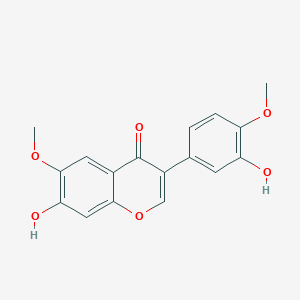
7-hydroxy-3-(3-hydroxy-4-methoxyphenyl)-6-methoxychromen-4-one
Computed by LexiChem 2.6.6 (PubChem release 2019.06.18)
InChI=1S/C17H14O6/c1-21-14-4-3-9(5-12(14)18)11-8-23-15-7-13(19)16(22-2)6-10(15)17(11)20/h3-8,18-19H,1-2H3
Computed by InChI 1.0.5 (PubChem release 2019.06.18)
BYNYZQQDQIQLSO-UHFFFAOYSA-N
Computed by InChI 1.0.5 (PubChem release 2019.06.18)
COC1=C(C=C(C=C1)C2=COC3=CC(=C(C=C3C2=O)OC)O)O
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C17H14O6
Computed by PubChem 2.1 (PubChem release 2019.06.18)
- 3aR-(3alpha)-isomer of odoratin
- geigerinin
- odoratin
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
314.29 g/mol
Reference
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Property Name
XLogP3-AA
Property Value
2.4
Reference
Computed by XLogP3 3.0 (PubChem release 2019.06.18)
Property Name
Hydrogen Bond Donor Count
Property Value
2
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Hydrogen Bond Acceptor Count
Property Value
6
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Rotatable Bond Count
Property Value
3
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Exact Mass
Property Value
314.07903816 Da
Reference
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Property Name
Monoisotopic Mass
Property Value
314.07903816 Da
Reference
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Property Name
Topological Polar Surface Area
Property Value
85.2 Ų
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Heavy Atom Count
Property Value
23
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
476
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2010.01.29)
Polyketides [PK] -> Flavonoids [PK12] -> Isoflavonoids [PK1205]
MoNA ID
MS Category
Experimental
MS Type
LC-MS
MS Level
MS2
Precursor Type
[M+H]+
Precursor m/z
315.086
Instrument
Maxis II HD Q-TOF Bruker
Ionization Mode
positive
Top 5 Peaks
315.086823 100
300.063873 37.13
316.090088 13.54
243.064911 12.38
167.034851 8.93
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Similar Compounds (2D)
Similar Conformers (3D)
Same Count
PubMed Count
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=BYNYZQQDQIQLSO-UHFFFAOYSA-N
The LOTUS Initiative for Open Natural Products Research: frozen dataset union wikidata (with metadata) | DOI:10.5281/zenodo.5794106
- ChEBI
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/LOTUS Treehttps://lotus.naturalproducts.net/
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- Japan Chemical Substance Dictionary (Nikkaji)
- KNApSAcK Species-Metabolite Database
- Natural Product Activity and Species Source (NPASS)
- LIPID MAPSLipid Classificationhttps://www.lipidmaps.org/
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/licenseNCGC00385697-01!7-hydroxy-3-(3-hydroxy-4-methoxyphenyl)-6-methoxychromen-4-onehttps://mona.fiehnlab.ucdavis.edu/spectra/browse?query=exists(compound.metaData.name:%27InChIKey%27%20and%20compound.metaData.value:%27BYNYZQQDQIQLSO-UHFFFAOYSA-N%27)
- Metabolomics Workbench
- SpectraBase7-HYDROXY-3-(3-HYDROXY-4-METHOXYPHENYL)-6-METHOXY-4H-1-BENZOPYRAN-4-ONEhttps://spectrabase.com/spectrum/8OuMGZVF6cx4',6-Dimethoxy-7,3'-dihydroxyflavonehttps://spectrabase.com/spectrum/JsDUw3zuHtM
- Springer Nature
- Wikidata
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.html
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 463906881https://pubchem.ncbi.nlm.nih.gov/substance/463906881
CONTENTS