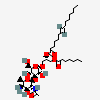Lipoteichoic Acid
PubChem CID
137349712
Molecular Formula
Synonyms
- Lipoteichoic acid
- 56411-57-5
- [(2S)-1-[(2S,3R,4S,5R,6R)-4-[(2S,3R,4S,5R,6R)-3-acetamido-5-amino-4-hydroxy-6-methyloxan-2-yl]oxy-3,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-3-heptanoyloxypropan-2-yl] (E)-pentadec-7-enoate
- Teichoic acid I
- LTA-1
Molecular Weight
775.0 g/mol
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Dates
- Create:2019-03-12
- Modify:2025-01-18
Description
Lipoteichoic acid has been reported in Streptococcus pyogenes with data available.
Lipoteichoic Acid is a biologically active lipopolysaccharide-type component of the Gram-negative bacterial cell wall. Lipoteichoic acid (LTA) may bind to cell surfaces non-specifically, through interactions with phospholipids, or specifically, through interactions with CD14 or toll-like receptors (TLR), and then be internalized. Binding to TLR2 may activate the NFkappaB pathway, mitogen-activated protein kinase (MAPK) signaling pathways and phosphoinositide 3-kinase (PI3K) activity. This can result in increased expression of pro-inflammatory cytokines and both pro- and anti-apoptotic genes and may stimulate expression of the immunosuppressive receptor programmed cell death protein 1 (PD-1; PDCD1; CD279). Increased expression of PD-1 promotes binding to its ligands, programmed cell death-1 ligand 1 (PD-L1) or 2 (PD-L2), which stimulates production of the anti-inflammatory cytokine interleukin-10 (IL-10) and leads to inhibition of CD4-positive T-cell proliferation and activity. Additionally, LTA also may induce cancer cell proliferation in certain susceptible tumor cells while having an inhibitory effect on proliferation of other cancer cells. Lastly, LTA levels in patient samples may also be correlated with inflammatory damage during acute bacterial infections.
Chemical Structure Depiction
Conformer generation is disallowed since too many atoms, too flexible
[(2S)-1-[(2S,3R,4S,5R,6R)-4-[(2S,3R,4S,5R,6R)-3-acetamido-5-amino-4-hydroxy-6-methyloxan-2-yl]oxy-3,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-3-heptanoyloxypropan-2-yl] (E)-pentadec-7-enoate
Computed by LexiChem 2.6.6 (PubChem release 2019.06.18)
InChI=1S/C39H70N2O13/c1-5-7-9-11-12-13-14-15-16-17-18-20-22-31(45)52-28(24-49-30(44)21-19-10-8-6-2)25-50-39-36(48)37(34(46)29(23-42)53-39)54-38-33(41-27(4)43)35(47)32(40)26(3)51-38/h14-15,26,28-29,32-39,42,46-48H,5-13,16-25,40H2,1-4H3,(H,41,43)/b15-14+/t26-,28-,29-,32+,33-,34-,35+,36-,37+,38+,39+/m1/s1
Computed by InChI 1.0.5 (PubChem release 2019.06.18)
PANDRCFROUDETH-YLSOAJEOSA-N
Computed by InChI 1.0.5 (PubChem release 2019.06.18)
CCCCCCC/C=C/CCCCCC(=O)O[C@@H](CO[C@@H]1[C@@H]([C@H]([C@@H]([C@H](O1)CO)O)O[C@H]2[C@@H]([C@H]([C@H]([C@H](O2)C)N)O)NC(=O)C)O)COC(=O)CCCCCC
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C39H70N2O13
Computed by PubChem 2.1 (PubChem release 2019.06.18)
- lipoteichoic acid
- LTA-1
- LTA-2
- teichoic acid I
- Lipoteichoic acid
- 56411-57-5
- [(2S)-1-[(2S,3R,4S,5R,6R)-4-[(2S,3R,4S,5R,6R)-3-acetamido-5-amino-4-hydroxy-6-methyloxan-2-yl]oxy-3,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-3-heptanoyloxypropan-2-yl] (E)-pentadec-7-enoate
- Teichoic acid I
- LTA-1
- LTA-2
- Lipoteichoic aCld
- ((2S)-1-((2S,3R,4S,5R,6R)-4-((2S,3R,4S,5R,6R)-3-acetamido-5-amino-4-hydroxy-6-methyloxan-2-yl)oxy-3,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl)oxy-3-heptanoyloxypropan-2-yl) (E)-pentadec-7-enoate
- (2s)-1-({3-O-[2-(Acetylamino)-4-Amino-2,4,6-Trideoxy-Beta-D-Galactopyranosyl]-Alpha-D-Glucopyranosyl}oxy)-3-(Heptanoyloxy)propan-2-Yl (7z)-Pentadec-7-Enoate
- Lipoteichoic acid from Bacillus subtilis
- DTXSID1040534
- CHEBI:28640
- (2s)-1-((3-O-(2-(Acetylamino)-4-Amino-2,4,6-Trideoxy-Beta-D-Galactopyranosyl)-Alpha-D-Glucopyranosyl)oxy)-3-(Heptanoyloxy)propan-2-Yl (7z)-Pentadec-7-Enoate
- DA-75031
- (sn-Gro-1-P)n->6Glc-beta1->6Glc-beta1->3acyl2Gro
- (sn-Gro-1-P)25->6Glc-alpha1->2Glc-alpha1->3acyl2Gro
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
775.0 g/mol
Reference
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Property Name
XLogP3-AA
Property Value
4.7
Reference
Computed by XLogP3 3.0 (PubChem release 2019.06.18)
Property Name
Hydrogen Bond Donor Count
Property Value
6
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Hydrogen Bond Acceptor Count
Property Value
14
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Rotatable Bond Count
Property Value
29
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Exact Mass
Property Value
774.48779029 Da
Reference
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Property Name
Monoisotopic Mass
Property Value
774.48779029 Da
Reference
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Property Name
Topological Polar Surface Area
Property Value
226 Ų
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Heavy Atom Count
Property Value
54
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
1080
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
11
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
1
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2019.01.04)
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Parent, Connectivity Count
Similar Compounds (2D)
Similar Conformers (3D)
Same Count
The LOTUS Initiative for Open Natural Products Research: frozen dataset union wikidata (with metadata) | DOI:10.5281/zenodo.5794106
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/Teichoic acid, lipo-https://commonchemistry.cas.org/detail?cas_rn=56411-57-5
- Comparative Toxicogenomics Database (CTD)LICENSEIt is to be used only for research and educational purposes. Any reproduction or use for commercial purpose is prohibited without the prior express written permission of NC State University.http://ctdbase.org/about/legal.jsplipoteichoic acidhttps://ctdbase.org/detail.go?type=chem&acc=C009900
- Therapeutic Target Database (TTD)Lipoteichoic acidhttps://idrblab.net/ttd/data/drug/details/D00UVU
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/Lipoteichoic acidhttps://www.wikidata.org/wiki/Q104396002LOTUS Treehttps://lotus.naturalproducts.net/
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuse
- Protein Data Bank in Europe (PDBe)
- WikidataLipoteichoic acidhttps://www.wikidata.org/wiki/Q104396002
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmllipoteichoic acidhttps://www.ncbi.nlm.nih.gov/mesh/67009900
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
CONTENTS