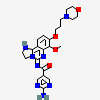
Copanlisib
- Copanlisib
- 1032568-63-0
- BAY 80-6946
- BAY-80-6946
- Aliqopa
- Create:2019-01-15
- Modify:2024-12-27
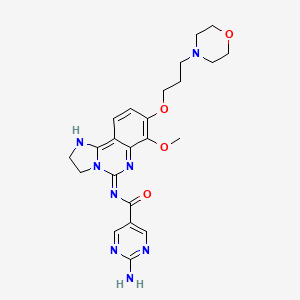
- 2-amino-N-(7-methoxy-8-(3-morpholinopropoxy)-2,3-dihydroimidazo(1,2-c)quinazolin-4-yl)pyrimidine-5-carboxamide
- Aliqopa
- BAY 80-6946
- copanlisib
- Copanlisib
- 1032568-63-0
- BAY 80-6946
- BAY-80-6946
- Aliqopa
- BAY80-6946
- BAY 80-6946 (Copanlisib)
- WI6V529FZ9
- 2-amino-N-(7-methoxy-8-(3-morpholinopropoxy)-2,3-dihydroimidazo[1,2-c]quinazolin-5-yl)pyrimidine-5-carboxamide
- 2-Amino-N-[2,3-dihydro-7-methoxy-8-[3-(4-morpholinyl)propoxy]imidazo[1,2-c]quinazolin-5-yl]-5-pyrimidinecarboxamide
- Copanlisib (BAY 80-6946)
- 2-amino-N-[7-methoxy-8-(3-morpholin-4-ylpropoxy)-2,3-dihydro-1H-imidazo[1,2-c]quinazolin-5-ylidene]pyrimidine-5-carboxamide
- 2-amino-N-[7-methoxy-8-(3-morpholin-4-ylpropoxy)-2,3-dihydroimidazo[1,2-c]quinazolin-5-yl]pyrimidine-5-carboxamide
- Copanlisib [INN]
- copanlisibum
- UNII-WI6V529FZ9
- 2-AMINO-N-(7-METHOXY-8-(3-MORPHOLIN-4-YLPROPOXY)-2,3-DIHYDROIMIDAZO(1,2-C)QUINAZOLIN-5-YL)PYRIMIDINE-5-CARBOXAMIDE
- Copanlisib tris-HCl
- MFCD18633201
- Copanlisib (Standard)
- COPANLISIB [MI]
- Copanlisib (USAN/INN)
- Copanlisib [USAN:INN]
- COPANLISIB [USAN]
- COPANLISIB [WHO-DD]
- Copanlisib(BAY80-6946)
- GTPL7875
- SCHEMBL1655478
- BAY 80-6946; Copanlisib
- BAY-80-6946 tris-HCl
- Copanlisib; BAY-80-6946
- CHEMBL3218576
- SCHEMBL13084037
- BAY 80-6946(Copanlisib)?
- DTXSID00145728
- CHEBI:173077
- PZBCKZWLPGJMAO-UHFFFAOYSA-N
- BCP04754
- EX-A2005
- BDBM50204093
- HY-15346R
- NSC760443
- NSC800076
- NSC809693
- NSC816437
- s2802
- AKOS025290222
- BAY-806946
- CS-0741
- DB12483
- NSC-760443
- NSC-800076
- NSC-809693
- NSC-816437
- PB22956
- VS-0128
- NCGC00346457-01
- NCGC00346457-02
- NCGC00346457-04
- NCGC00346457-05
- 2-amino-N-(7-methoxy-8-(3-morpholinopropoxy)-2,3-dihydroimidazo(1,2-c)quinazolin-4-yl)pyrimidine-5-carboxamide
- AC-28438
- BAY 80-6946?
- BC164810
- HY-15346
- NS00072920
- D10867
- BRD-K24666289-001-02-3
- BRD-K24666289-001-04-9
- Q19903876
- 2-AMINO-N-(7-METHOXY-8-(3-(MORPHOLIN-4-YL)PROPOXY)-2,3-DIHYDROIMIDAZO(1,2-C)QUINAZOLIN-5-YL(PYRIMIDINE-5-CARBOXAMIDE
- 2-amino-N-[(5E)-7-methoxy-8-[3-(morpholin-4-yl)propoxy]-1H,2H,3H-imidazo[1,2-c]quinazolin-5-ylidene]pyrimidine-5-carboxamide
- 2-amino-N-[7-methoxy-8-(3-morpholin-4-ylpropoxy)-2,3-dihydroimidazo[1.2-c]quinazolin-5-yl]pyrimidine-5-carboxamide
- 2-amino-N-{7-methoxy-8-[3-(morpholin-4-yl)propoxy]-1H,2H,3H-imidazo[1,2-c]quinazolin-5-ylidene}pyrimidine-5-carboxamide
- 2-amino-N-{7-methoxy-8-[3-(morpholin-4-yl)propoxy]-2,3-dihydroimidazo[1,2-c]quinazolin-5-yl}pyrimidine-5-carboxamide
- 5-PYRIMIDINECARBOXAMIDE, 2-AMINO-N-(2,3-DIHYDRO-7-METHOXY-8-(3-(4-MORPHOLINYL)PROPOXY)IMIDAZO(1,2-C)QUINAZOLIN-5-YL)-
Not Classified
Reported as not meeting GHS hazard criteria by 1 of 1 companies. For more detailed information, please visit ECHA C&L website.
◉ Summary of Use during Lactation
Copanlisib has been removed from the US market. No information is available on the clinical use of copanlisib during breastfeeding. Because copanlisib's half-life is about 39 hours, it might accumulate in the infant. The manufacturer recommends that breastfeeding be discontinued during copanlisib therapy and for 1 month after the last dose.
◉ Effects in Breastfed Infants
Relevant published information was not found as of the revision date.
◉ Effects on Lactation and Breastmilk
Relevant published information was not found as of the revision date.
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=MWYDSXOGIBMAET-UHFFFAOYSA-N
- ChEBI
- FDA Pharm ClassesLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingFDA Pharmacological Classificationhttps://www.fda.gov/ForIndustry/DataStandards/StructuredProductLabeling/ucm162549.htm
- Open TargetsLICENSEDatasets generated by the Open Targets Platform are freely available for download.https://platform-docs.opentargets.org/licence
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- Comparative Toxicogenomics Database (CTD)LICENSEIt is to be used only for research and educational purposes. Any reproduction or use for commercial purpose is prohibited without the prior express written permission of NC State University.http://ctdbase.org/about/legal.jsp
- Drug Gene Interaction database (DGIdb)LICENSEThe data used in DGIdb is all open access and where possible made available as raw data dumps in the downloads section.http://www.dgidb.org/downloads
- IUPHAR/BPS Guide to PHARMACOLOGYLICENSEThe Guide to PHARMACOLOGY database is licensed under the Open Data Commons Open Database License (ODbL) https://opendatacommons.org/licenses/odbl/. Its contents are licensed under a Creative Commons Attribution-ShareAlike 4.0 International License (http://creativecommons.org/licenses/by-sa/4.0/)https://www.guidetopharmacology.org/about.jsp#licenseGuide to Pharmacology Target Classificationhttps://www.guidetopharmacology.org/targets.jsp
- Therapeutic Target Database (TTD)
- ChemIDplusCopanlisib [USAN:INN]https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=1032568630ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSToxCompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-notice2-amino-N-(7-methoxy-8-(3-morpholinopropoxy)-2,3-dihydroimidazo[1,2-c]quinazolin-5-yl)pyrimidine-5-carboxamidehttps://echa.europa.eu/substance-information/-/substanceinfo/100.260.2752-amino-N-(7-methoxy-8-(3-morpholinopropoxy)-2,3-dihydroimidazo[1,2-c]quinazolin-5-yl)pyrimidine-5-carboxamide (EC: 825-220-1)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/268444
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- Drugs and Lactation Database (LactMed)
- Drugs@FDALICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- EU Clinical Trials Register
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citing
- Japan Chemical Substance Dictionary (Nikkaji)
- NIPH Clinical Trials Search of Japan
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/CopanlisibNORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- Protein Data Bank in Europe (PDBe)
- Springer Nature
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- WikidataCopanlisibhttps://www.wikidata.org/wiki/Q126607135
- WikipediaCopanlisibhttps://en.wikipedia.org/wiki/Copanlisib
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.html
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlUSP drug classificationhttp://www.genome.jp/kegg-bin/get_htext?br08302.kegAnatomical Therapeutic Chemical (ATC) classificationhttp://www.genome.jp/kegg-bin/get_htext?br08303.kegTarget-based classification of drugshttp://www.genome.jp/kegg-bin/get_htext?br08310.keg
- WHO Anatomical Therapeutic Chemical (ATC) ClassificationLICENSEUse of all or parts of the material requires reference to the WHO Collaborating Centre for Drug Statistics Methodology. Copying and distribution for commercial purposes is not allowed. Changing or manipulating the material is not allowed.https://www.whocc.no/copyright_disclaimer/
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 471048692https://pubchem.ncbi.nlm.nih.gov/substance/471048692