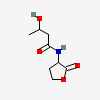
3-hydroxy-N-(2-oxooxolan-3-yl)butanamide
PubChem CID
130846
Molecular Formula
Synonyms
- 912545-51-8
- 3-hydroxy-N-(2-oxooxolan-3-yl)butanamide
- 126049-72-7
- HBHSL
- 3-Hydroxy-butanoyl-DL-homoserine lactone
Molecular Weight
187.19 g/mol
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Dates
- Create:2005-08-08
- Modify:2025-01-18
Chemical Structure Depiction
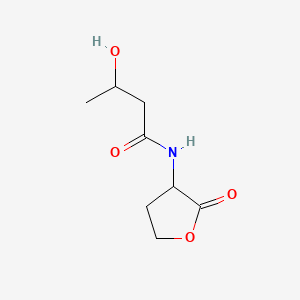
SVG Image

IUPAC Condensed
deamino-xiThr-DL-Hsl
Sequence
XX
HELM
PEPTIDE1{[*C(=O)CC(C)O |$_R2;;;;;;$|].[*NC1CCOC1=O |$_R1;;;;;;;$|]}$$$$
IUPAC
deamino-threonyl-DL-homoserine lactone
3-hydroxy-N-(2-oxooxolan-3-yl)butanamide
Computed by Lexichem TK 2.7.0 (PubChem release 2021.10.14)
InChI=1S/C8H13NO4/c1-5(10)4-7(11)9-6-2-3-13-8(6)12/h5-6,10H,2-4H2,1H3,(H,9,11)
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
FIXDIFPJOFIIEC-UHFFFAOYSA-N
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
CC(CC(=O)NC1CCOC1=O)O
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C8H13NO4
Computed by PubChem 2.2 (PubChem release 2021.10.14)
126049-72-7
- 3-hydroxy-C4-HSL
- HBHL, Vibrio harveyi autoinducer
- HBHSL
- N-(3-hydroxybutanoyl)homoserine lactone
- N-(beta-hydroxybutyryl)homoserine lactone
- N3-HBHL
- 912545-51-8
- 3-hydroxy-N-(2-oxooxolan-3-yl)butanamide
- 126049-72-7
- HBHSL
- 3-Hydroxy-butanoyl-DL-homoserine lactone
- 3-Hydroxy-N-(2-oxotetrahydrofuran-3-yl)butanamide
- N3-Hbhl
- Hbhl, vibrio harveyi autoinducer
- MFCD24520804
- N-(3-Hydroxybutanoyl)homoserine lactone
- N-(beta-Hydroxybutyryl)homoserine lactone
- SCHEMBL4399583
- 3-hydroxy-C4-homoserine lactone
- DTXSID00925385
- 3-Hydroxy-butanoyl-DL-homoserinelactonemin98%
- G89696
- 3-Hydroxy-N-(2-oxooxolan-3-yl)butanimidic acid
- Butanamide, 3-hydroxy-N-(tetrahydro-2-oxo-3-furanyl)-
- 3-Hydroxy-butanoyl-DL-homoserine lactone, min. 98% (3-OH-C4-DL-Hsl)
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
187.19 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
XLogP3-AA
Property Value
-0.1
Reference
Computed by XLogP3 3.0 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Donor Count
Property Value
2
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Acceptor Count
Property Value
4
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Rotatable Bond Count
Property Value
3
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Exact Mass
Property Value
187.08445790 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Monoisotopic Mass
Property Value
187.08445790 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Topological Polar Surface Area
Property Value
75.6 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Heavy Atom Count
Property Value
13
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
216
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
2
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
Spectra ID
Ionization Mode
Negative
Top 5 Peaks
84.0457 100
57.0344 31.01
Spectra ID
Ionization Mode
Negative
Top 5 Peaks
84.0457 100
112.0408 51.83
142.0514 17.54
80.0502 5.76
68.0511 3.66
Accession ID
Authors
BGC, Helmholtz Zentrum Muenchen
Instrument
maXis plus UHR-ToF-MS, Bruker Daltonics
Instrument Type
LC-ESI-QTOF
MS Level
MS2
Ionization Mode
POSITIVE
Ionization
ESI
Collision Energy
10
Fragmentation Mode
CID
Column Name
BEH C18 1.7um, 2.1x100mm, Waters
Retention Time
0.913 min
Precursor m/z
188.0917
Precursor Adduct
[M+H]+
Top 5 Peaks
102.0546 999
188.0917 436
87.0435 69
74.0231 21
170.0815 18
License
CC BY
Accession ID
Authors
BGC, Helmholtz Zentrum Muenchen
Instrument
maXis plus UHR-ToF-MS, Bruker Daltonics
Instrument Type
LC-ESI-QTOF
MS Level
MS2
Ionization Mode
POSITIVE
Ionization
ESI
Collision Energy
20
Fragmentation Mode
CID
Column Name
BEH C18 1.7um, 2.1x100mm, Waters
Retention Time
0.913 min
Precursor m/z
188.0917
Precursor Adduct
[M+H]+
Top 5 Peaks
102.0546 999
74.0593 872
84.0437 199
87.0434 189
69.0331 87
License
CC BY
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Parent, Connectivity Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=FIXDIFPJOFIIEC-UHFFFAOYSA-N
- ChemIDplusN-(3-Hydroxybutanoyl)homoserine lactonehttps://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0126049727ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSTox3-Hydroxy-N-(2-oxooxolan-3-yl)butanimidic acidhttps://comptox.epa.gov/dashboard/DTXSID00925385CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citing(3R)-3-Hydroxy-N-[(3S)-2-oxooxolan-3-yl]butanamidehttp://www.hmdb.ca/metabolites/HMDB0243607HMDB0243607_msms_2244550https://hmdb.ca/metabolites/HMDB0243607#spectra
- Japan Chemical Substance Dictionary (Nikkaji)
- MassBank Europe3-hydroxy-C4-homoserine lactonehttps://massbank.eu/MassBank/Result.jsp?inchikey=FIXDIFPJOFIIEC-UHFFFAOYSA-N
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- Wikidata3-Hydroxy-N-(2-oxooxolan-3-yl)butanimidic acidhttps://www.wikidata.org/wiki/Q82899700
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlN-(3-hydroxybutanoyl)homoserine lactonehttps://www.ncbi.nlm.nih.gov/mesh/67062185
- PATENTSCOPE (WIPO)SID 393824146https://pubchem.ncbi.nlm.nih.gov/substance/393824146
CONTENTS