Carboxyprimaquine
PubChem CID
127542
Molecular Formula
Synonyms
- 77229-68-6
- Carboxyprimaquine
- 4-((6-Methoxyquinolin-8-yl)amino)pentanoic acid
- 4-[(6-methoxyquinolin-8-yl)amino]pentanoic acid
- Carboxy Primaquine
Molecular Weight
274.31 g/mol
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Dates
- Create:2005-03-27
- Modify:2025-01-18
Description
Carboxyprimaquine is an organonitrogen compound and an organooxygen compound. It is functionally related to a gamma-amino acid.
Chemical Structure Depiction
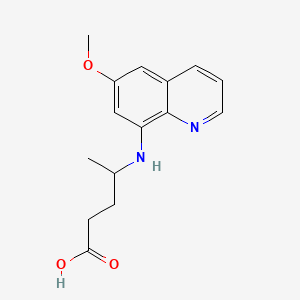
4-[(6-methoxyquinolin-8-yl)amino]pentanoic acid
Computed by Lexichem TK 2.7.0 (PubChem release 2024.11.20)
InChI=1S/C15H18N2O3/c1-10(5-6-14(18)19)17-13-9-12(20-2)8-11-4-3-7-16-15(11)13/h3-4,7-10,17H,5-6H2,1-2H3,(H,18,19)
Computed by InChI 1.07.0 (PubChem release 2024.11.20)
KIMKJIXTIWKABF-UHFFFAOYSA-N
Computed by InChI 1.07.0 (PubChem release 2024.11.20)
CC(CCC(=O)O)NC1=C2C(=CC(=C1)OC)C=CC=N2
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C15H18N2O3
Computed by PubChem 2.2 (PubChem release 2024.11.20)
77229-68-6
- 8-(3-carboxy-1-methylpropylamino)-6-methoxyquinoline
- carboxyprimaquine
- CMPAMOQ
- 77229-68-6
- Carboxyprimaquine
- 4-((6-Methoxyquinolin-8-yl)amino)pentanoic acid
- 4-[(6-methoxyquinolin-8-yl)amino]pentanoic acid
- Carboxy Primaquine
- Cmpamoq
- NZZ7G26XIV
- 8-(3-Carboxy-1-methylpropylamino)-6-methoxyquinoline
- UNII-NZZ7G26XIV
- 4-[(6-methoxy-8-quinolyl)amino]valeric acid
- 4-[(6-Methoxy-8-quinolinyl)amino]pentanoic acid
- Pentanoic acid, 4-((6-methoxy-8-quinolinyl)amino)-
- Pentanoic acid, 4-[(6-methoxy-8-quinolinyl)amino]-
- 4-((6-METHOXY-8-QUINOLINYL)AMINO)PENTANOIC ACID
- 4-((6-methoxy-8-quinolyl)amino)valeric acid
- MFCD00598907
- SCHEMBL5644378
- DTXSID40891676
- CHEBI:165855
- CDA22968
- AKOS015940349
- SB71032
- AC-27752
- NS00014469
- PRI_275.1391_17.2
- 4-[(6-Methoxy-8-quinolinyl)amino]pentanoic acid #
- 8-(3-carboxy-1-methylpropylamino)-6-methoxy-quinoline
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
274.31 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Property Name
XLogP3-AA
Property Value
2.4
Reference
Computed by XLogP3 3.0 (PubChem release 2024.11.20)
Property Name
Hydrogen Bond Donor Count
Property Value
2
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Hydrogen Bond Acceptor Count
Property Value
5
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Rotatable Bond Count
Property Value
6
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Exact Mass
Property Value
274.13174244 Da
Reference
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Property Name
Monoisotopic Mass
Property Value
274.13174244 Da
Reference
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Property Name
Topological Polar Surface Area
Property Value
71.5 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Heavy Atom Count
Property Value
20
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
324
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
1
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
NIST Number
128319
Library
Main library
Total Peaks
150
m/z Top Peak
201
m/z 2nd Highest
274
m/z 3rd Highest
215
Thumbnail
Spectra ID
Ionization Mode
Positive
Top 5 Peaks
132.0681 100
55.0543 54.58
160.063 19.05
175.0863 16.01
131.0603 14.11
Spectra ID
Ionization Mode
Positive
Top 5 Peaks
55.0542 100
175.0865 97.67
132.0682 78.24
160.0631 44.61
83.0491 29.99
Accession ID
Authors
R. Gulde, E. Schymanski, K. Fenner, Department of Environmental Chemistry, Eawag
Instrument
Q Exactive Plus Orbitrap Thermo Scientific
Instrument Type
LC-ESI-QFT
MS Level
MS2
Ionization Mode
POSITIVE
Ionization
ESI
Collision Energy
15 (nominal)
Fragmentation Mode
HCD
Column Name
Atlantis T3 3um, 3.0x150mm, Waters with guard column
Retention Time
16.8 min
Precursor m/z
275.139
Precursor Adduct
[M+H]+
Top 5 Peaks
257.1284 999
187.0962 36
60.0808 26
83.0855 19
57.0699 16
License
CC BY
Reference
Gulde, R.; Meier, U.; Schymanski, E. L.; Kohler, H.-P. E.; Helbling, D. E.; Derrer, S.; Rentsch, D.; Fenner, K. Systematic Exploration of Biotransformation Reactions of Amine-Containing Micropollutants in Activated Sludge. Environmental Science & Technology 2016, 50 (6), 2908-20. DOI:10.1021/acs.est.5b05186
Accession ID
Authors
R. Gulde, E. Schymanski, K. Fenner, Department of Environmental Chemistry, Eawag
Instrument
Q Exactive Plus Orbitrap Thermo Scientific
Instrument Type
LC-ESI-QFT
MS Level
MS2
Ionization Mode
POSITIVE
Ionization
ESI
Collision Energy
30 (nominal)
Fragmentation Mode
HCD
Column Name
Atlantis T3 3um, 3.0x150mm, Waters with guard column
Retention Time
16.8 min
Precursor m/z
275.139
Precursor Adduct
[M+H]+
Top 5 Peaks
257.1284 999
175.0866 102
101.0598 71
57.0699 53
83.0856 48
License
CC BY
Reference
Gulde, R.; Meier, U.; Schymanski, E. L.; Kohler, H.-P. E.; Helbling, D. E.; Derrer, S.; Rentsch, D.; Fenner, K. Systematic Exploration of Biotransformation Reactions of Amine-Containing Micropollutants in Activated Sludge. Environmental Science & Technology 2016, 50 (6), 2908-20. DOI:10.1021/acs.est.5b05186
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Stereo Count
Same Isotope Count
Same Parent, Connectivity Count
Same Parent, Stereo Count
Same Parent, Isotope Count
Same Parent, Exact Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
PubMed Count
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=KIMKJIXTIWKABF-UHFFFAOYSA-N
- ChEBICarboxyprimaquinehttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:165855
- ChemIDplus8-(3-Carboxy-1-methylpropylamino)-6-methoxyquinolinehttps://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0077229686ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingCARBOXYPRIMAQUINEhttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/NZZ7G26XIV
- EPA DSSTox4-[(6-methoxyquinolin-8-yl)amino]pentanoic acidhttps://comptox.epa.gov/dashboard/DTXSID40891676CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citing4-((6-Methoxyquinolin-8-yl)amino)pentanoic acidhttp://www.hmdb.ca/metabolites/HMDB0247386HMDB0247386_msms_2246595https://hmdb.ca/metabolites/HMDB0247386#spectra
- Japan Chemical Substance Dictionary (Nikkaji)
- MassBank Europe
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- Metabolomics Workbench
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-lawCarboxyprimaquinehttp://www.nist.gov/srd/nist1a.cfm
- PharmGKBLICENSEPharmGKB data are subject to the Creative Commons Attribution-ShareALike 4.0 license (https://creativecommons.org/licenses/by-sa/4.0/).https://www.pharmgkb.org/page/policiescarboxyprimaquinehttps://www.pharmgkb.org/chemical/PA166326661
- Springer Nature
- Wikidata4-[(6-methoxyquinolin-8-yl)amino]pentanoic acidhttps://www.wikidata.org/wiki/Q82003473
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.html8-(3-carboxy-1-methylpropylamino)-6-methoxyquinolinehttps://www.ncbi.nlm.nih.gov/mesh/67035150
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 389987670https://pubchem.ncbi.nlm.nih.gov/substance/389987670
CONTENTS