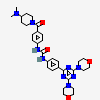
Gedatolisib
PubChem CID
44516953
Molecular Formula
Synonyms
- Gedatolisib
- 1197160-78-3
- PKI-587
- PF-05212384
- 1-(4-(4-(Dimethylamino)piperidine-1-carbonyl)phenyl)-3-(4-(4,6-dimorpholino-1,3,5-triazin-2-yl)phenyl)urea
Molecular Weight
615.7 g/mol
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Dates
- Create:2009-12-22
- Modify:2025-01-18
Description
Gedatolisib has been used in trials studying the basic science and treatment of Neoplasm, Ovary Cancer, Breast Cancer, Advanced Cancer, and Endometrial Cancer, among others.
Gedatolisib is an agent targeting the phosphatidylinositol 3 kinase (PI3K) and mammalian target of rapamycin (mTOR) in the PI3K/mTOR signaling pathway, with potential antineoplastic activity. Upon intravenous administration, gedatolisib inhibits both PI3K and mTOR kinases, which may result in apoptosis and growth inhibition of cancer cells overexpressing PI3K/mTOR. Activation of the PI3K/mTOR pathway promotes cell growth, survival, and resistance to chemotherapy and radiotherapy; mTOR, a serine/threonine kinase downstream of PI3K, may also be activated independent of PI3K.
GEDATOLISIB is a small molecule drug with a maximum clinical trial phase of III (across all indications) and has 8 investigational indications.
Chemical Structure Depiction
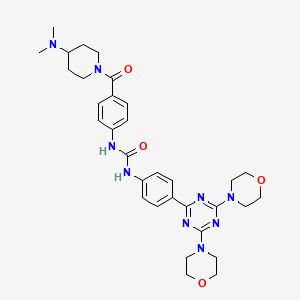
1-[4-[4-(dimethylamino)piperidine-1-carbonyl]phenyl]-3-[4-(4,6-dimorpholin-4-yl-1,3,5-triazin-2-yl)phenyl]urea
Computed by Lexichem TK 2.7.0 (PubChem release 2021.10.14)
InChI=1S/C32H41N9O4/c1-38(2)27-11-13-39(14-12-27)29(42)24-5-9-26(10-6-24)34-32(43)33-25-7-3-23(4-8-25)28-35-30(40-15-19-44-20-16-40)37-31(36-28)41-17-21-45-22-18-41/h3-10,27H,11-22H2,1-2H3,(H2,33,34,43)
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
DWZAEMINVBZMHQ-UHFFFAOYSA-N
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
CN(C)C1CCN(CC1)C(=O)C2=CC=C(C=C2)NC(=O)NC3=CC=C(C=C3)C4=NC(=NC(=N4)N5CCOCC5)N6CCOCC6
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C32H41N9O4
Computed by PubChem 2.2 (PubChem release 2021.10.14)
- 1-(4-((4-(Dimethylamino)piperidin-1-yl)carbonyl)phenyl)-3-(4-(4,6-dimorpholin-4-yl-1,3,5-triazin-2-yl)phenyl)urea
- gedatolisib
- PF-05212384
- PKI 587
- PKI-587
- PKI587
- Gedatolisib
- 1197160-78-3
- PKI-587
- PF-05212384
- 1-(4-(4-(Dimethylamino)piperidine-1-carbonyl)phenyl)-3-(4-(4,6-dimorpholino-1,3,5-triazin-2-yl)phenyl)urea
- PKI 587
- PKI587
- PF 05212384
- 1-[4-[4-(dimethylamino)piperidine-1-carbonyl]phenyl]-3-[4-(4,6-dimorpholin-4-yl-1,3,5-triazin-2-yl)phenyl]urea
- CHEMBL592445
- Gedatolisib (PF-05212384, PKI-587)
- 96265TNH2R
- Urea, N-[4-[[4-(dimethylamino)-1-piperidinyl]carbonyl]phenyl]-N'-[4-(4,6-di-4-morpholinyl-1,3,5-triazin-2-yl)phenyl]-
- PKI-587; PF-05212384
- Urea, N-(4-((4-(dimethylamino)-1-piperidinyl)carbonyl)phenyl)-N'-(4-(4,6-di-4-morpholinyl-1,3,5-triazin-2-yl)phenyl)-
- PF-05212384 (PKI-587)
- 1-(4-((4-(dimethylamino)piperidin-1-yl)carbonyl)phenyl)-3-(4-(4,6-dimorpholin-4-yl-1,3,5-triazin-2-yl)phenyl)urea
- Gedatolisib [USAN:INN]
- UNII-96265TNH2R
- 1-(4-{[4-(Dimethylamino)piperidin-1-yl]carbonyl}phenyl)-3-[4-(4,6-dimorpholin-4-yl-1,3,5-triazin-2-yl)phenyl]urea
- MFCD16875679
- PK 587
- PK 1587
- Gedatolisib(PKI-587)
- GEDATOLISIB [INN]
- Gedatolisib (USAN/INN)
- GEDATOLISIB [USAN]
- Gedatolisib (PKI-587)
- Gedatolisib(PKI-587)?
- SCHEMBL32393
- GEDATOLISIB [WHO-DD]
- N-[4-[[4-(Dimethylamino)-1-piperidinyl]carbonyl]phenyl]-N'-[4-[4,6-di(4-morpholinyl)-1,3,5-triazin-2-yl]phenyl]urea
- GTPL7940
- DTXSID40152557
- EX-A028
- DWZAEMINVBZMHQ-UHFFFAOYSA-N
- HMS3748M11
- BCP01986
- BDBM50308135
- NSC758256
- NSC801014
- s2628
- AKOS005766013
- CCG-264662
- CS-0449
- DB11896
- FE-0013
- NSC-758256
- NSC-801014
- SB16571
- SDCCGSBI-0654417.P001
- NCGC00370777-01
- NCGC00370777-04
- NCGC00370777-09
- NCGC00370777-10
- AC-31519
- HY-10681
- NS00072612
- PKI-587,PF-05212384
- D10635
- J-004182
- J-523339
- BRD-K07955840-001-02-3
- BRD-K07955840-001-03-1
- BRD-K07955840-001-04-9
- Q27077788
- PKI-587|1197160-78-3|PKI587|PKI 587
- 3-{4-[4,6-BIS(MORPHOLIN-4-YL)-1,3,5-TRIAZIN-2-YL]PHENYL}-1-{4-[4-(DIMETHYLAMINO)PIPERIDINE-1-CARBONYL]PHENYL}UREA
- N-(4-((4-(DIMETHYLAMINO)-1-PIPERIDINYL)CARBONYL)PHENYL)-N'-(4-(4,6-DIMORPHOLIN-4-YL-1,3,5-TRIAZIN-2-YL)PHENYL)UREA
- N-{4-[4,6-bis(morpholin-4-yl)-1,3,5-triazin-2-yl]phenyl}-N'-{4-[4-(dimethylamino)piperidine-1-carbonyl]phenyl}urea
- VL1
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
615.7 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
XLogP3-AA
Property Value
2.4
Reference
Computed by XLogP3 3.0 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Donor Count
Property Value
2
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Acceptor Count
Property Value
10
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Rotatable Bond Count
Property Value
7
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Exact Mass
Property Value
615.32815082 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Monoisotopic Mass
Property Value
615.32815082 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Topological Polar Surface Area
Property Value
128 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Heavy Atom Count
Property Value
45
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
913
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
Pharmaceuticals -> Listed in ZINC15
S55 | ZINC15PHARMA | Pharmaceuticals from ZINC15 | DOI:10.5281/zenodo.3247749
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Parent, Connectivity Count
Same Parent, Exact Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
Protein Kinase Inhibitors
Agents that inhibit PROTEIN KINASES. (See all compounds classified as Protein Kinase Inhibitors.)
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=DWZAEMINVBZMHQ-UHFFFAOYSA-N
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/
- ChemIDplusGedatolisib [USAN:INN]https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=1197160783ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DrugBankLICENSECreative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode)https://www.drugbank.ca/legal/terms_of_useGedatolisibhttps://www.drugbank.ca/drugs/DB11896
- EPA DSSToxCompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- Comparative Toxicogenomics Database (CTD)LICENSEIt is to be used only for research and educational purposes. Any reproduction or use for commercial purpose is prohibited without the prior express written permission of NC State University.http://ctdbase.org/about/legal.jsp
- Drug Gene Interaction database (DGIdb)LICENSEThe data used in DGIdb is all open access and where possible made available as raw data dumps in the downloads section.http://www.dgidb.org/downloadsGEDATOLISIBhttps://www.dgidb.org/drugs/ncit:C91732
- IUPHAR/BPS Guide to PHARMACOLOGYLICENSEThe Guide to PHARMACOLOGY database is licensed under the Open Data Commons Open Database License (ODbL) https://opendatacommons.org/licenses/odbl/. Its contents are licensed under a Creative Commons Attribution-ShareAlike 4.0 International License (http://creativecommons.org/licenses/by-sa/4.0/)https://www.guidetopharmacology.org/about.jsp#licenseGuide to Pharmacology Target Classificationhttps://www.guidetopharmacology.org/targets.jsp
- Therapeutic Target Database (TTD)
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- Open TargetsLICENSEDatasets generated by the Open Targets Platform are freely available for download.https://platform-docs.opentargets.org/licence
- EU Clinical Trials Register
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citingGedatolisibhttp://www.hmdb.ca/metabolites/HMDB0256629
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlTarget-based classification of drugshttp://www.genome.jp/kegg-bin/get_htext?br08310.keg
- Metabolomics Workbench
- NIPH Clinical Trials Search of Japan
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/GedatolisibNORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- PharosLICENSEData accessed from Pharos and TCRD is publicly available from the primary sources listed above. Please respect their individual licenses regarding proper use and redistribution.https://pharos.nih.gov/aboutgedatolisibhttps://pharos.nih.gov/ligands/B42ALPJNZD2W
- Protein Data Bank in Europe (PDBe)
- RCSB Protein Data Bank (RCSB PDB)LICENSEData files contained in the PDB archive (ftp://ftp.wwpdb.org) are free of all copyright restrictions and made fully and freely available for both non-commercial and commercial use. Users of the data should attribute the original authors of that structural data.https://www.rcsb.org/pages/policies
- Springer Nature
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- Wikidatagedatolisibhttps://www.wikidata.org/wiki/Q27077788
- WikipediaGedatolisibhttps://en.wikipedia.org/wiki/Gedatolisib
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlgedatolisibhttps://www.ncbi.nlm.nih.gov/mesh/67549060Protein Kinase Inhibitorshttps://www.ncbi.nlm.nih.gov/mesh/68047428
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 391933997https://pubchem.ncbi.nlm.nih.gov/substance/391933997
- NCBI
CONTENTS