Octanoate
PubChem CID
119389
Molecular Formula
Synonyms
- octanoate
- Caprylate
- capryloate
- n-octanoate
- caprilate
Molecular Weight
143.20 g/mol
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Parent Compound
Dates
- Create:2004-09-16
- Modify:2025-01-18
Description
Octanoate is a straight-chain saturated fatty acid anion that is the conjugate base of octanoic acid (caprylic acid); believed to block adipogenesis. It has a role as a human metabolite and a Saccharomyces cerevisiae metabolite. It is a fatty acid anion 8:0 and a straight-chain saturated fatty acid anion. It is a conjugate base of an octanoic acid.
Chemical Structure Depiction
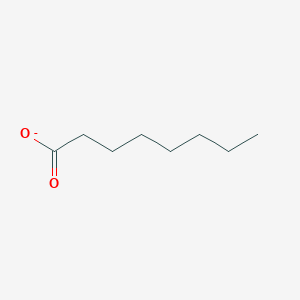
octanoate
Computed by LexiChem 2.6.6 (PubChem release 2019.06.18)
InChI=1S/C8H16O2/c1-2-3-4-5-6-7-8(9)10/h2-7H2,1H3,(H,9,10)/p-1
Computed by InChI 1.0.5 (PubChem release 2019.06.18)
WWZKQHOCKIZLMA-UHFFFAOYSA-M
Computed by InChI 1.0.5 (PubChem release 2019.06.18)
CCCCCCCC(=O)[O-]
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C8H15O2-
Computed by PubChem 2.1 (PubChem release 2019.06.18)
- caprylic acid
- caprylic acid, 14C-labeled
- caprylic acid, aluminum salt
- caprylic acid, ammonia salt
- caprylic acid, barium salt
- caprylic acid, cadmium salt
- caprylic acid, calcium salt
- caprylic acid, cesium salt
- caprylic acid, chromium(+2) salt
- caprylic acid, cobalt salt
- caprylic acid, copper salt
- caprylic acid, copper(+2) salt
- caprylic acid, iridum(+3) salt
- caprylic acid, iron(+3) salt
- caprylic acid, lanthanum(+3) salt
- caprylic acid, lead(+2) salt
- caprylic acid, lithium salt
- caprylic acid, manganese salt
- caprylic acid, nickel(+2) salt
- caprylic acid, potassium salt
- caprylic acid, ruthenium(+3) salt
- caprylic acid, sodium salt
- caprylic acid, sodium salt, 11C-labeled
- caprylic acid, tin salt
- caprylic acid, tin(+2) salt
- caprylic acid, zinc salt
- caprylic acid, zirconium salt
- caprylic acid, zirconium(+4) salt
- lithium octanoate
- octanoate
- octanoic acid
- sodium caprylate
- sodium octanoate
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
143.20 g/mol
Reference
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Property Name
XLogP3
Property Value
3.7
Reference
Computed by XLogP3 3.0 (PubChem release 2019.06.18)
Property Name
Hydrogen Bond Donor Count
Property Value
0
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Hydrogen Bond Acceptor Count
Property Value
2
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Rotatable Bond Count
Property Value
5
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Exact Mass
Property Value
143.107204717 Da
Reference
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Property Name
Monoisotopic Mass
Property Value
143.107204717 Da
Reference
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Property Name
Topological Polar Surface Area
Property Value
40.1 Ų
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Heavy Atom Count
Property Value
10
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
-1
Reference
Computed by PubChem
Property Name
Complexity
Property Value
83.7
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2011.04.04)
1D NMR Spectra
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Parent, Connectivity Count
Same Parent, Exact Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
Same Count
PubMed Count
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=WWZKQHOCKIZLMA-UHFFFAOYSA-M
WormJam Metabolites Local CSV for MetFrag | DOI:10.5281/zenodo.3403364
WormJam: A consensus C. elegans Metabolic Reconstruction and Metabolomics Community and Workshop Series, Worm, 6:2, e1373939, DOI:10.1080/21624054.2017.1373939
- BindingDBLICENSEAll data curated by BindingDB staff are provided under the Creative Commons Attribution 3.0 License (https://creativecommons.org/licenses/by/3.0/us/).https://www.bindingdb.org/rwd/bind/info.jsp
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/
- ChemIDplusChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSToxCompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- ChEBI
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- Crystallography Open Database (COD)LICENSEAll data in the COD and the database itself are dedicated to the public domain and licensed under the CC0 License. Users of the data should acknowledge the original authors of the structural data.https://creativecommons.org/publicdomain/zero/1.0/
- ECI Group, LCSB, University of Luxembourgoctanoic acid
- Natural Product Activity and Species Source (NPASS)
- FooDBLICENSEFooDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (FooDB) and the original publication.https://foodb.ca/aboutoctanoatehttps://foodb.ca/compounds/FDB031069
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citing
- Japan Chemical Substance Dictionary (Nikkaji)
- NMRShiftDB
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/OctanoateNORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- Rhea - Annotated Reactions DatabaseLICENSERhea has chosen to apply the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/). This means that you are free to copy, distribute, display and make commercial use of the database in all legislations, provided you credit (cite) Rhea.https://www.rhea-db.org/help/license-disclaimer
- SpectraBaseOctanoate anionhttps://spectrabase.com/spectrum/DzAClEm7DzR
- Wikidata
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmloctanoic acidhttps://www.ncbi.nlm.nih.gov/mesh/67031492
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 389693646https://pubchem.ncbi.nlm.nih.gov/substance/389693646
CONTENTS

 CID 379 (Octanoic Acid)
CID 379 (Octanoic Acid)