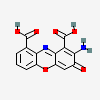
Cinnabarinic acid
PubChem CID
114918
Molecular Formula
Synonyms
- Cinnabarinic acid
- 606-59-7
- Cinnavalininate
- 2-amino-3-oxo-3h-phenoxazine-1,9-dicarboxylic acid
- 2-amino-3-oxophenoxazine-1,9-dicarboxylic acid
Molecular Weight
300.22 g/mol
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Dates
- Create:2005-06-24
- Modify:2025-01-18
Description
Cinnavalininate is a phenoxazine.
Cinnavalininate is a metabolite found in or produced by Escherichia coli (strain K12, MG1655).
Chemical Structure Depiction
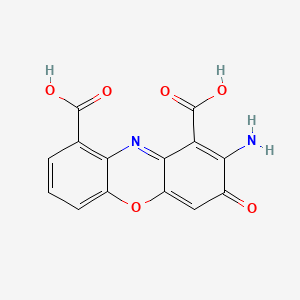
2-amino-3-oxophenoxazine-1,9-dicarboxylic acid
Computed by Lexichem TK 2.7.0 (PubChem release 2021.10.14)
InChI=1S/C14H8N2O6/c15-10-6(17)4-8-12(9(10)14(20)21)16-11-5(13(18)19)2-1-3-7(11)22-8/h1-4H,15H2,(H,18,19)(H,20,21)
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
FSBKJYLVDRVPTK-UHFFFAOYSA-N
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
C1=CC(=C2C(=C1)OC3=CC(=O)C(=C(C3=N2)C(=O)O)N)C(=O)O
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C14H8N2O6
Computed by PubChem 2.2 (PubChem release 2021.10.14)
cinnabarinic acid
- Cinnabarinic acid
- 606-59-7
- Cinnavalininate
- 2-amino-3-oxo-3h-phenoxazine-1,9-dicarboxylic acid
- 2-amino-3-oxophenoxazine-1,9-dicarboxylic acid
- 2XYB6EX2PG
- 2-Amino-3H-phenoxazin-one-1,9-dicarboxylic acid
- 2-Amino-3-oxo-3H-phenoxazin-1,9-dicarboxylic acid
- CHEBI:3715
- CHEMBL2322655
- 3H-Phenoxazin-1,9-dicarboxylic acid, 2-amino-3-oxo-
- Cinnabaric acid
- C14H8N2O6
- Cinnavalininic acid
- CYNNABARINNIC ACID
- UNII-2XYB6EX2PG
- Cinnabarinic acid (Standard)
- 3H-Phenoxazine-1,9-dicarboxylic acid, 2-amino-3-oxo-
- SCHEMBL11888793
- DTXSID30209408
- BCPP000278
- HY-W011417R
- BDBM50428068
- HB0195
- MFCD18379297
- Cinnabarinic Acid, >=98% (HPLC)
- AKOS024457983
- BCP9000532
- CS-W012133
- HY-W011417
- DA-62330
- C05640
- G60963
- Q27106176
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
300.22 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
XLogP3-AA
Property Value
0.8
Reference
Computed by XLogP3 3.0 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Donor Count
Property Value
3
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Acceptor Count
Property Value
8
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Rotatable Bond Count
Property Value
2
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Exact Mass
Property Value
300.03823598 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Monoisotopic Mass
Property Value
300.03823598 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Topological Polar Surface Area
Property Value
139 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Heavy Atom Count
Property Value
22
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
675
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
Solid
0.604
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Parent, Connectivity Count
Same Parent, Exact Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
PubMed Count
Tryptophan metabolism
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=FSBKJYLVDRVPTK-UHFFFAOYSA-N
WormJam Metabolites Local CSV for MetFrag | DOI:10.5281/zenodo.3403364
WormJam: A consensus C. elegans Metabolic Reconstruction and Metabolomics Community and Workshop Series, Worm, 6:2, e1373939, DOI:10.1080/21624054.2017.1373939
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/Cinnabarinic acidhttps://commonchemistry.cas.org/detail?cas_rn=606-59-7
- ChemIDplusChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSToxCinnabarinic acidhttps://comptox.epa.gov/dashboard/DTXSID30209408CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingCINNABARINIC ACIDhttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/2XYB6EX2PG
- ChEBICinnavalininatehttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:3715
- E. coli Metabolome Database (ECMDB)
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- Comparative Toxicogenomics Database (CTD)LICENSEIt is to be used only for research and educational purposes. Any reproduction or use for commercial purpose is prohibited without the prior express written permission of NC State University.http://ctdbase.org/about/legal.jspcinnabarinic acidhttps://ctdbase.org/detail.go?type=chem&acc=C000483
- ECI Group, LCSB, University of LuxembourgCinnavalininate
- Natural Product Activity and Species Source (NPASS)Cinnabarinic Acidhttps://bidd.group/NPASS/compound.php?compoundID=NPC8467
- FooDBLICENSEFooDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (FooDB) and the original publication.https://foodb.ca/aboutCinnavalininatehttps://foodb.ca/compounds/FDB023302
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citingCinnavalininatehttp://www.hmdb.ca/metabolites/HMDB0004078
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.html
- Metabolomics Workbench
- PharosLICENSEData accessed from Pharos and TCRD is publicly available from the primary sources listed above. Please respect their individual licenses regarding proper use and redistribution.https://pharos.nih.gov/about2-amino-3-oxo-3H-phenoxazine-1,9-dicarboxylic acidhttps://pharos.nih.gov/ligands/T5R3LAU1LLNS
- Springer Nature
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- Wikidatacinnavalininatehttps://www.wikidata.org/wiki/Q27106176
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlcinnabarinic acidhttps://www.ncbi.nlm.nih.gov/mesh/67000483
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/LOTUS Treehttps://lotus.naturalproducts.net/
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 392812527https://pubchem.ncbi.nlm.nih.gov/substance/392812527
- NCBI
CONTENTS