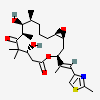
Epothilone
PubChem CID
10838895
Molecular Formula
Synonyms
- Epothilone
- SCHEMBL15195675
- (1S,3S,7R,10R,11S,12S,16R)-7,11-dihydroxy-8,8,10,12-tetramethyl-3-[(E)-1-(2-methyl-1,3-thiazol-4-yl)prop-1-en-2-yl]-4,17-dioxabicyclo[14.1.0]heptadecane-5,9-dione
Molecular Weight
493.7 g/mol
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Dates
- Create:2006-10-26
- Modify:2025-01-18
Description
A group of 16-member MACROLIDES which stabilize MICROTUBULES in a manner similar to PACLITAXEL. They were originally found in the myxobacterium Sorangium cellulosum, now renamed to Polyangium (MYXOCOCCALES).
Chemical Structure Depiction
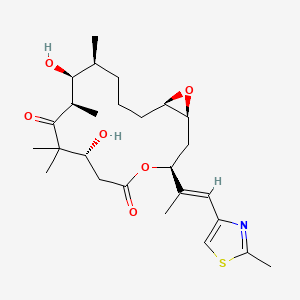
(1S,3S,7R,10R,11S,12S,16R)-7,11-dihydroxy-8,8,10,12-tetramethyl-3-[(E)-1-(2-methyl-1,3-thiazol-4-yl)prop-1-en-2-yl]-4,17-dioxabicyclo[14.1.0]heptadecane-5,9-dione
Computed by Lexichem TK 2.7.0 (PubChem release 2024.11.20)
InChI=1S/C26H39NO6S/c1-14-8-7-9-19-21(32-19)11-20(15(2)10-18-13-34-17(4)27-18)33-23(29)12-22(28)26(5,6)25(31)16(3)24(14)30/h10,13-14,16,19-22,24,28,30H,7-9,11-12H2,1-6H3/b15-10+/t14-,16+,19+,20-,21-,22+,24-/m0/s1
Computed by InChI 1.07.0 (PubChem release 2024.11.20)
HESCAJZNRMSMJG-DEEJXTFZSA-N
Computed by InChI 1.07.0 (PubChem release 2024.11.20)
C[C@H]1CCC[C@@H]2[C@@H](O2)C[C@H](OC(=O)C[C@H](C(C(=O)[C@@H]([C@H]1O)C)(C)C)O)/C(=C/C3=CSC(=N3)C)/C
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C26H39NO6S
Computed by PubChem 2.2 (PubChem release 2024.11.20)
- Epothilon
- Epothilone
- Epothilones
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
493.7 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Property Name
XLogP3-AA
Property Value
4
Reference
Computed by XLogP3 3.0 (PubChem release 2024.11.20)
Property Name
Hydrogen Bond Donor Count
Property Value
2
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Hydrogen Bond Acceptor Count
Property Value
8
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Rotatable Bond Count
Property Value
2
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Exact Mass
Property Value
493.24980914 Da
Reference
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Property Name
Monoisotopic Mass
Property Value
493.24980914 Da
Reference
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Property Name
Topological Polar Surface Area
Property Value
137 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Heavy Atom Count
Property Value
34
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
770
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
7
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
1
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2019.01.04)
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Isotope Count
Same Parent, Connectivity Count
Same Parent, Isotope Count
Same Parent, Exact Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
Tubulin Modulators
Agents that interact with TUBULIN to inhibit or promote polymerization of MICROTUBULES. (See all compounds classified as Tubulin Modulators.)
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=HESCAJZNRMSMJG-DEEJXTFZSA-N
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- Comparative Toxicogenomics Database (CTD)LICENSEIt is to be used only for research and educational purposes. Any reproduction or use for commercial purpose is prohibited without the prior express written permission of NC State University.http://ctdbase.org/about/legal.jsp
- EU Clinical Trials Register
- Japan Chemical Substance Dictionary (Nikkaji)
- Springer Nature
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlEpothiloneshttps://www.ncbi.nlm.nih.gov/mesh/68034261Tubulin Modulatorshttps://www.ncbi.nlm.nih.gov/mesh/68050257
- PubChem
- PATENTSCOPE (WIPO)SID 389113337https://pubchem.ncbi.nlm.nih.gov/substance/389113337
CONTENTS