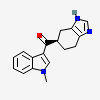
Ramosetron
PubChem CID
108000
Molecular Formula
Synonyms
- Ramosetron
- 132036-88-5
- Ramosetron [INN]
- (1-methylindol-3-yl)-[(5R)-4,5,6,7-tetrahydro-3H-benzimidazol-5-yl]methanone
- UNII-7ZRO0SC54Y
Molecular Weight
279.34 g/mol
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Dates
- Create:2005-06-24
- Modify:2025-01-11
Description
Ramosetron is a member of indoles.
Ramosetron is a serotonin 5-HT3 receptor antagonist commonly employed to treat nausea and vomiting, in addition to certain diarrheal conditions. It is believed to have higher potency and longer antiemetic action than other 1st generation 5-HT3 antagonists such as ondansetron. Currently, ramosetron is only approved for use Japan and in certain Southeast Asian countries.
Ramosetron is a selective serotonin (5-HT) receptor antagonist, with potential antiemetic activity. Upon administration, ramosetron selectively binds to and blocks the activity of 5-HT subtype 3 (5-HT3) receptors located in the vagus nerve terminal and in the vomiting center in the central nervous system (CNS), suppressing chemotherapy-induced nausea and vomiting.
Chemical Structure Depiction

(1-methylindol-3-yl)-[(5R)-4,5,6,7-tetrahydro-3H-benzimidazol-5-yl]methanone
Computed by Lexichem TK 2.7.0 (PubChem release 2021.10.14)
InChI=1S/C17H17N3O/c1-20-9-13(12-4-2-3-5-16(12)20)17(21)11-6-7-14-15(8-11)19-10-18-14/h2-5,9-11H,6-8H2,1H3,(H,18,19)/t11-/m1/s1
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
NTHPAPBPFQJABD-LLVKDONJSA-N
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
CN1C=C(C2=CC=CC=C21)C(=O)[C@@H]3CCC4=C(C3)NC=N4
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C17H17N3O
Computed by PubChem 2.2 (PubChem release 2021.10.14)
- 5-((1-methyl-3-indolyl)carbonyl)-4,5,6,7-tetrahydro-1H-benzimidazol
- Methanone, (1-methyl-1H-indol-3-yl)(4,5,6,7-tetrahydro-1H-benzimidazol-5-yl)-, monohydrochloride, (R)-
- Nasea
- ramosetron
- ramosetron hydrochloride
- YM 060
- YM-060
- YM060
- Ramosetron
- 132036-88-5
- Ramosetron [INN]
- (1-methylindol-3-yl)-[(5R)-4,5,6,7-tetrahydro-3H-benzimidazol-5-yl]methanone
- UNII-7ZRO0SC54Y
- Nor-YM 060
- 7ZRO0SC54Y
- Ramosetron (INN)
- RAMOSETRON [MI]
- RAMOSETRON [WHO-DD]
- CHEMBL1643895
- DTXSID0043842
- Methanone, (1-methyl-1H-indol-3-yl)[(6R)-4,5,6,7-tetrahydro-1H-benzimidazol-6-yl]-
- (R)-(1-Methyl-1H-indol-3-yl)(4,5,6,7-tetrahydro-1H-benzo[d]imidazol-6-yl)methanone
- ibsetron hydrochloride
- ramosetronum
- (1-methylindol-3-yl)-((5R)-4,5,6,7-tetrahydro-3H-benzimidazol-5-yl)methanone
- SCHEMBL16701
- GTPL2301
- DTXCID8023842
- CHEBI:135156
- NTHPAPBPFQJABD-LLVKDONJSA-N
- BDBM50334454
- (-)-(R)-1-Methylindol-3-yl 4,5,6,7-tetrahydro-5-benzimidazolylketone
- AKOS015896003
- DB09290
- NS00069945
- D08466
- Q2979523
- BRD-K85046107-003-01-2
- (-)-(R)-1-Methylindol-3-yl-4,5,6,7-tetrahydro-5-benzimidazolyl ketone
- (-)-(R)-1-Methylindol-3-yl-4,5,6,7-tetrahydro-5-benzimidazolyl ketone.
- 5-((1-methyl-3-indolyl)carbonyl)-4,5,6,7-tetrahydro-1H-benzimidazol
- (5R)-5-(1-methyl-1H-indole-3-carbonyl)-4,5,6,7-tetrahydro-1H-1,3-benzodiazole
- (R)-(-)-5-[(1-methyl-3-indolyl)carbonyl]-4,5,6,7-tetrahydrobenzimidazole
- METHANONE,(1-METHYL-1H-INDOL-3-YL)[(6R)-4,5,6,7-TETRAHYDRO-1H-BENZIMIDAZOL-6-YL]-
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
279.34 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
XLogP3-AA
Property Value
2.2
Reference
Computed by XLogP3 3.0 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Donor Count
Property Value
1
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Acceptor Count
Property Value
2
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Rotatable Bond Count
Property Value
2
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Exact Mass
Property Value
279.137162174 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Monoisotopic Mass
Property Value
279.137162174 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Topological Polar Surface Area
Property Value
50.7 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Heavy Atom Count
Property Value
21
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
413
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
1
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
Pharmaceuticals -> Listed in ZINC15
S55 | ZINC15PHARMA | Pharmaceuticals from ZINC15 | DOI:10.5281/zenodo.3247749
Instrument Name
Bio-Rad FTS
Technique
ATR-Film (MeCl2) (DuraSamplIR II)
Source of Spectrum
Forensic Spectral Research
Source of Sample
Cayman Chemical Company
Catalog Number
Free base of 15548
Lot Number
Free base of 0456487-4
Copyright
Copyright © 2014-2024 John Wiley & Sons, Inc. All Rights Reserved.
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Stereo Count
Same Isotope Count
Same Parent, Connectivity Count
Same Parent, Stereo Count
Same Parent, Isotope Count
Same Parent, Exact Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
For the treatment of nausea and vomiting and diarrhea-predominant irritable bowel syndrome in males.
Serotonin Antagonists
Drugs that bind to but do not activate serotonin receptors, thereby blocking the actions of serotonin or SEROTONIN RECEPTOR AGONISTS. (See all compounds classified as Serotonin Antagonists.)
Antiemetics
Drugs used to prevent NAUSEA or VOMITING. (See all compounds classified as Antiemetics.)
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/
- ChemIDplusChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DrugBankLICENSECreative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode)https://www.drugbank.ca/legal/terms_of_useRamosetronhttps://www.drugbank.ca/drugs/DB09290
- EPA DSSToxCompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- ChEBI
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- Open TargetsLICENSEDatasets generated by the Open Targets Platform are freely available for download.https://platform-docs.opentargets.org/licence
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- Drug Gene Interaction database (DGIdb)LICENSEThe data used in DGIdb is all open access and where possible made available as raw data dumps in the downloads section.http://www.dgidb.org/downloadsRAMOSETRONhttps://www.dgidb.org/drugs/ncit:C61921
- IUPHAR/BPS Guide to PHARMACOLOGYLICENSEThe Guide to PHARMACOLOGY database is licensed under the Open Data Commons Open Database License (ODbL) https://opendatacommons.org/licenses/odbl/. Its contents are licensed under a Creative Commons Attribution-ShareAlike 4.0 International License (http://creativecommons.org/licenses/by-sa/4.0/)https://www.guidetopharmacology.org/about.jsp#licenseGuide to Pharmacology Target Classificationhttps://www.guidetopharmacology.org/targets.jsp
- EU Clinical Trials Register
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlTarget-based classification of drugshttp://www.genome.jp/kegg-bin/get_htext?br08310.keg
- Metabolomics Workbench
- NIPH Clinical Trials Search of Japan
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/RamosetronNORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- Therapeutic Target Database (TTD)
- PharosLICENSEData accessed from Pharos and TCRD is publicly available from the primary sources listed above. Please respect their individual licenses regarding proper use and redistribution.https://pharos.nih.gov/about
- SpectraBase
- Wikidataramosetronhttps://www.wikidata.org/wiki/Q2979523
- WikipediaGlycineamide ribonucleotidehttps://en.wikipedia.org/wiki/Glycineamide_ribonucleotideRamosetronhttps://en.wikipedia.org/wiki/Ramosetron
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlSerotonin Antagonistshttps://www.ncbi.nlm.nih.gov/mesh/68012702Antiemeticshttps://www.ncbi.nlm.nih.gov/mesh/68000932
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- NCBI
CONTENTS