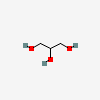
Glycerin
- C3H8O3
- CH2OH-CHOH-CH2OH
- glycerol
- glycerin
- 56-81-5
- PROPANE-1,2,3-TRIOL
- Glycerine
- Create:2004-09-16
- Modify:2025-01-18
 Polyglycerin-3 (monomer of); Tobacco Leaf (part of);
Polyglycerin-3 (monomer of); Tobacco Leaf (part of);  Polyglyceryl-3 Diisostearate (monomer of) ... View More ...
Polyglyceryl-3 Diisostearate (monomer of) ... View More ...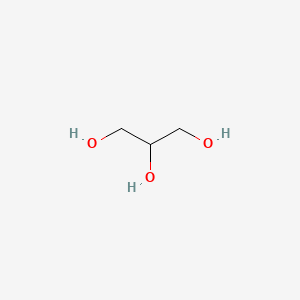
C3H8O3
CH2OH-CHOH-CH2OH
- 1,2,3-Propanetriol
- 1,2,3-Trihydroxypropane
- Glycerin
- Glycerine
- Glycerol
- glycerol
- glycerin
- 56-81-5
- PROPANE-1,2,3-TRIOL
- Glycerine
- 1,2,3-Propanetriol
- Trihydroxypropane
- Glyceritol
- Glycyl alcohol
- 1,2,3-trihydroxypropane
- Propanetriol
- Osmoglyn
- Glysanin
- Grocolene
- Glyrol
- Glycerinum
- Ophthalgan
- Vitrosupos
- Dagralax
- Glycerin, anhydrous
- Glycerin, synthetic
- Synthetic glycerin
- Moon
- Synthetic glycerine
- Glycerolum
- Optim
- Star
- Glycerin mist
- Incorporation factor
- 90 Technical glycerine
- Citifluor AF 2
- Glycerin (mist)
- Glicerol
- Bulbold
- Cristal
- Glicerina [DCIT]
- Glycerine mist
- Tryhydroxypropane
- Caswell No. 469
- Glycerin,anhydrous
- Glycerin [JAN]
- FEMA No. 2525
- Clyzerin, wasserfrei
- Propanetriol (VAN)
- Glicerina
- Monoctanoin Component D
- Glycerin, natural
- CCRIS 2295
- Glicerol [INN-Spanish]
- Glycerolum [INN-Latin]
- HSDB 492
- EPA Pesticide Chemical Code 063507
- Pricerine 9091
- NSC 9230
- AI3-00091
- Clyzerin, wasserfrei [German]
- Emery 916
- Glyzerin
- Oelsuess
- di-o-tolylphenylphosphine
- BRN 0635685
- UNII-PDC6A3C0OX
- Collyrium Fresh-Eye Drops
- PDC6A3C0OX
- 1,2,3-trihydroxypropanol
- NSC-9230
- EINECS 200-289-5
- IFP
- Glycerol [INN]
- Glycerol 85%
- DTXSID9020663
- CHEBI:17754
- INS-422
- NSC9230
- Glycerin [USP:JAN]
- DYNASTIN 7
- MFCD00004722
- M 314429
- PZN 7474853
- DTXCID40662
- INS NO.422
- E-422
- EC 200-289-5
- Glycerol (INN)
- 2-PROPANOL, 1,3-DIHYDROXY-
- 101662-08-2
- 144086-03-3
- 8013-25-0
- NCGC00090950-03
- Glycerin (USP:JAN)
- Diacylglycerol(35:0)
- GLYCERIN (II)
- GLYCERIN [II]
- Glycerin base
- Glicerol (INN-Spanish)
- Glycerolum (INN-Latin)
- GLYCEROL (MART.)
- GLYCEROL [MART.]
- GLYCERIN (USP-RS)
- GLYCERIN [USP-RS]
- 107283-02-3
- 153050-05-6
- 1H-Thieno[3,4-d]iMidazole-4-pentanaMide, hexahydro-2-oxo-N-[6-oxo-6-(2-propenylaMino)hexyl]-, (3aS,4
- GLYCEROL (EP IMPURITY)
- GLYCEROL [EP IMPURITY]
- GLYCEROL (EP MONOGRAPH)
- GLYCEROL [EP MONOGRAPH]
- GLYCERIN (USP MONOGRAPH)
- GLYCERIN [USP MONOGRAPH]
- Glycerol; Propane-1,2,3-Triol
- Heterochromatin-specific nonhistone chromosomal protein HP-1
- DAG 31:3
- DAG 35:0
- DAG(35:0)
- Glycerol, ACS reagent, >=99.5%
- MAG(20:2)
- MAG(20:3)
- Glyceol Opthalgan
- DG 31:3
- DG 35:0
- MG 17:1
- MG 20:2
- MG 20:3
- CAS-56-81-5
- DG(31:3)
- DG(35:0)
- GOL
- MG(20:2)
- MG(20:3)
- Mackstat H 66
- RG-S
- glicerolo
- keragel
- Glycylalcohol
- Neutracett
- Antizom
- Glyceol
- Inclear
- keragelT
- Monagra
- Olsubeta
- GlycerinAdult
- Artifical tears
- MedPrideLemon
- MeWrinkle
- myCellCare
- OceanTears
- RepoveSolutionA
- RepoveSolutionB
- Sayak
- Glycerin Cream
- Glycerin Toner
- Leader Glycerin
- Lemon Glycerin
- Lemon Glycerine
- Moisture Cream
- Peptide Easter
- Apple Bubble
- Baby Essence
- Biotoc Regen
- D-glycerol
- L-glycerol
- Massage Cream
- Mermaid Tears
- Regener-Eyes
- Renewal Serum
- The Prestige
- Ato Pollen
- Avedana Glycerin
- Derma Glide
- Dynarex Hydrogel
- Glycerin Essence
- Illite Whitening
- Scar Serum
- DooBony Mosrepel
- Regener-EyesPRO
- WeTTrust Gold
- Yeo Danbi
- Absolute PPC
- Derma First Duo
- Fedora Cinderella
- Galentic Hydrogel
- Glycerin Laxative
- My Lovely Butts
- Man Power Pack
- CP Medi theraphy
- Derma Honey Mask
- Flex Power Cream
- ZNSP Ampoule
- Cellpium Shinyline
- CP Bebe wash
- Oasis Tears PF
- Oriental Fermented
- Regener-EyesLITE
- ZNSP Repair
- Bliss GVSChildren
- GMDANSU Ampoule
- OR Serum
- Black Paint Rubar
- Bliss GVS
- Premiu Serum Mask
- Bio DNA Serum
- OASIS Tears
- 1,3-Propanetriol
- CP Bebe
- CP Medi
- EGF-FGF Ampoule
- HYDRA Rose Mask
- Queen Perfume Hair
- Glycerol, ultrapure
- Anti Wrinkle effect
- Dr Deep BodyLotion
- Glycerin USP grade
- OASIS Tears Plus
- all-in-one cleanser
- Deer Velvet Ampoule
- Glycerin Suppository
- Necbody All in one
- Obeo 7way Moisture
- Topwin Speedy Enema
- DR.SERSE Serum
- Glycerin 25%
- Hum CC
- O Sha mpoo Premium
- Dr Deep AtodeepSoap
- Dr Deep Deep Fresh
- Hyalucollagen Essence
- HUITOMI
- Im V-Tox
- MORAN
- Elikin Aqua MaskPack
- La face All in One
- The Cica Serum Plus
- AC Clear Sheet Mask
- CIZAR First Calming
- diacylglycerol 31:3
- diacylglycerol 35:0
- Glizigen Gel Intimate
- Glyzigen Gel Intimate
- Hyalucollagen Moisture
- Moisture Essence Mist
- Natural Cocktail Pack
- Wrinkle Power Filling
- 11003-00-2
- AMAZING AMPOULE
- Clapiel Vita C Serum
- CORRECT COMBO
- EYE CLEANSING
- Glycerin, concentrated
- HUITOMI SERUM
- LANBELLE LANBIO
- LIFT EYECREAM
- MAKEUP REMOVER
- REMEMBER SERUM
- RESURFACE MASK
- TANGERINE HAND
- AMULDY KIDS
- HUITOMI PACK
- ING AMPOULE
- MIRACLE ATO
- NYMPHSYN EYE
- TMC-A Anti Hairloss
- YOZUM SECRET
- Coca-Glycerine Control
- DERM-APPLY
- FORMULA H
- 1,3-Trihydroxypropane
- 90 Technical glycerin
- BURZANGMURY GOLD
- Dr Deep NaturalShampoo
- HAIR REGENERATOR
- Leader GlycerinLaxative
- VITALISING TONIC
- AC Control Daily Mask
- Adult Glycerin Laxative
- aewajin Derma Hydrocell
- ATO ALL
- Biotrue Hydration Boost
- DNA COUPLE MASK
- Emery 912
- Eyeganics Organic Tears
- Glycerol-Saline Control
- Glycerol-Saline Diluent
- MEDI HYDRO DP
- Medpride Lemon Glycerin
- monoacylglycerol 17:1
- monoacylglycerol 20:2
- monoacylglycerol 20:3
- Rayderm Hydrating Toner
- VITAMIN C SERUM
- Whimela Shining Implant
- AC ALL
- Anti Wrinkle Eye Serum
- Avedana GlycerinLaxative
- diacylglycerol(31:3)
- LANBELLE Clear Toner
- TEN TEN CELL
- Co2 Polymer Mask Pack
- COLDS RUNNY NOSE
- FIRMING Camellia Mask
- G3S AMPOULE
- GLOWING Marigold Mask
- MIANJU MASK PACK
- GLYCERIN [HSDB]
- GLYCEROL [FHFI]
- Anti Wrinkle Effect Eye
- Cicatrix Skin Protectant
- Glizigen Skin Protectant
- Infant Glycerin Laxative
- Lemon GlycerineSwabsticks
- Longevity Recovery Serum
- Origin Horse Sheet Mask
- Rubelli Wine Foot Packs
- Rubelli Wine Hand Packs
- Soriso Bright Aqua Pack
- Vision Clarity Eye Drop
- Walgreens Adult Glycerin
- Zenibell Snail Intensive
- BouMatic Syst X Premium
- Dr Deep BodyLotion Mini
- GLYCEROL [MI]
- HAHNEMANN SNEEZING
- MERDEVIE FIRECREAM
- NON-SHEET SERUMST
- NYMPHSYN MAXIMIZER
- Querencia CREAMfor Nail
- Vitamin B5 Velvet Mask
- 2X FIRST ESSENCE
- GLYCERIN [VANDF]
- MEDI HYDRO DP BB
- monoacylglycerol(17:1)
- monoacylglycerol(20:2)
- monoacylglycerol(20:3)
- Oasis TEARS PF PLUS
- Ultra Soothing Mask 1pc
- Effective Hair Strengthen
- Lemon Glycerin Swabsticks
- Refining Pore Clear Pack
- Elikin Whitening MaskPack
- GLYCERINUM [HPUS]
- NYMPHSYN FACE MASK
- SELLA NATURAL BABY
- SELLA NATURAL HAIR
- E 422
- Glycerin (JP17/USP)
- OASIS TearsLubricant Eye
- Anti Wrinkle Effect Ample
- Anti Wrinkle Hand Essence
- Stratuscare Adult Glycerin
- Ultra Moisturizing Essence
- Wrinkle Repair Sheet Mask
- beeRX Cold Sore Treatment
- Hwang Geum-San Gold Hair
- KOREAN CYPRESS HAIR
- LEBODY FACE RENEWAL
- MEDI HYDRO DP MIST
- REBORNCELL M-CLINIC
- bmse000184
- bmse000807
- bmse000856
- CHEMBL692
- Correway Personal Lubricant
- Dead Skin Perfect Cleanser
- Df-2
- GLYCEROL [WHO-DD]
- GLYCEROL [WHO-IP]
- Hyalucollagen Moisture Skin
- Jena Cell VL Volume Lift
- MolMap_000024
- Pediatric Glycerin Laxative
- Tomatox Magic Massage Pack
- Walgreens Children Glycerin
- CIZAR First Calming Toner
- Fall in Rub (Love) Hand
- Glycerol, >=99.5%
- Glycerol, biochemical grade
- LANBELLE LANS TAMANU
- MEDI HYDRO DP TONER
- MELATONIN SHEET MASK
- Moisture hydrogel mask pack
- OERBEUA CALMING MASK
- OERBEUA FIRMING MASK
- OERBEUA RADIANT MASK
- OERBEUA SHINING MASK
- PEPTICOL 5S AMPOULE
- Rayderm Re-generating serum
- Soriso bright aqua Moisture
- WELLAGE Vital Nio Enrich
- ATONO2 Oxygen Baby Cream
- Rayderm Re-generating Cream
- WELLAGE 4D Gold Essence
- WLN: Q1YQ1Q
- ALA-C Cell clinic solution
- Hyalucollagen Moisture Toner
- Mefactory Piggy Peeling Pad
- Aloevera Soothing Gel 98%
- ATONO2 Oxygen Bubble Foam
- LANBELLE EGF FGF Ampule
- LANBELLE VITAMIN CELL
- OERBEUA CLEARING MASK
- OERBEUA MOISTURE MASK
- OERBEUA SOOTHING MASK
- SISEUNDEUSI BLESS LIP
- 2-hydroxylpropane-1,3-diol
- Glycerol, LR, >=98%
- Renovating Regenerating Serum
- Truezyme Healing Multi Tonic
- Truezyme Healing Scalp Tonic
- ALL IN ONE DUAL MASK
- Elikin Anti Wrinkle MaskPack
- Glycerol, analytical standard
- HAIR TREATMENT PREMIUM
- NOBLESSE HOMME REFRESH
- OERBEUA HYDRATING MASK
- OERBEUA SMOOTHING MASK
- SISEUNDEUSI ULTRA SPOT
- SUN KILLING GRAPEFRUIT
- TAMNAMO ESSENTIAL HAIR
- WELLAGE POST PROCEDURE
- Zenibell Snail IntensiveCream
- 4-01-00-02751 (Beilstein Handbook Reference)
- Anti aging hydrogel mask pack
- CVS Pharmacy GlycerinLaxative
- Inner Beauty Cleansing Tissue
- Optimel Manuka Dry Eye Drops
- Optimel Manuka Forte Eye Gel
- Pick Me Pad Azulene Moisture
- Pick Me Pad Make-up Remover
- Glycerol min 98%, anhydrous
- LIMPIADOR DE OJOS WEYE
- Miracle of the Mose Hair Bar
- OERBEUA ENERGIZING MASK
- OERBEUA MATTIFYING MASK
- OERBEUA NOURISHING MASK
- ATONO2 Oxygen Baby Bath and
- ATONO2 Oxygen Pure Bath and
- Glycerin mist (ACGIH,OSHA)
- Glycerol, >=99% (GC)
- Pesticide Code: 063507.
- Zenibell Snail Intensive Toner
- AN ADC EGF WRINKLE EYE
- DESMAQUILLANTE OJOS WEYE
- Dr Deep Enrich NourishingCream
- GLYCERIN [ORANGE BOOK]
- INIbebe Organic Soapberry Wash
- NYMPHSYN INTENSIVE SERUM
- PURE HYDROGEL MASK PACK
- By Pharmicell Lab The Prestige
- Dr Deep Propolis Synergy Serum
- GTPL5195
- H2 365 CLEAN
- Perfect V Lifting Premium Mask
- Prevention Oncology Mouth Rinse
- QSPL 181
- Remember Hydrosol Double Effect
- Dr Deep ALL in ONE Cleansing
- Dr.NUELL Mugwort Shaking Pack
- LANBELLE MASQUE DE GENIE
- PURE HYDROGEL APPLE ZONE
- QUEEN79 NOBLE GOLDCREAM
- SISEUNDEUSI SCALP ESSENCE
- THE BANHAM Peony 365 plus
- ULTRA REJUVE 7S AMPOULE
- Walgreens Adult GlycerinLaxative
- Dr.NUELL Brightening Mask Pack
- Dr.NUELL True Deep Mosturizing
- Glycerol, AR, >=99.5%
- GUERISON ALL IN ONE TOCX
- Leaders Mediu Glossy Honey Pack
- MIRU Oriental Whitening Ampoule
- NATURAL SAVON 4IN1 PLUS
- Pentrioxido sulfurico glycerincol
- WELLAGE Intensive Repair Serum
- BURZANGMURY GOLD TREATMENT
- Fedora ORIENTAL SPA AMPOULE
- Glycerol, >99%, FCC, FG
- Glycerol, technical grade, 95%
- InstaMix G Emollient Concentrate
- LANBELLE ANTI-WRINKLE EYE
- Ruby ReBorn Transfusion DNA Kit
- SPEARMINT Aromatherapy Mask27g
- A06AG04
- A06AX01
- BUENO ANTI-WRINKLE PEPTIDE
- DTXSID10991197
- Fedora FIRMING INTENSIVE EYE
- FIND BICHON CLEANSING MASK
- Glycerol, ultrapure, HPLC Grade
- Jena Cell Super cell serum PLUS
- JNH a-Clear Propolis Spot Serum
- QUEEN79 NOBLE GOLD SERUM
- QUICK CLEANSING DRY TISSUE
- 2w97
- ATONO2 Oxygen Baby Soothing Gel
- CHEBI:131416
- CHEBI:178017
- CHEBI:189439
- CHEBI:232593
- CHEBI:232596
- CHEBI:232600
- GLYCEROL 85% [WHO-DD]
- Glycerol, ACS reagent, 99.5%
- Glycerol, Molecular Biology Grade
- GLYCEROLUM [WHO-IP LATIN]
- Glyzigen Single Dose Intimate Gel
- InstaMix Emollient Concentrate EC
- isLeaf Clinic Lime Cleansing Foam
- Pororo Friends Moistful Mask Pack
- Truezyme Baby ShampooAndBody Wash
- 3 en 1 Cleanser. Deep Cleansing
- Clear Eyes Advanced Dry and Itchy
- Dr. B Anti Pollution Bubble Mask
- DR. GLODERM TABRX MOISTURE
- GLYCERIN,ANHYDROUS [VANDF]
- Pharmakon1600-01300020
- Silkriller 3D Gold Pure Rich Gel
- TAMHADA Synergy Moisturizing Mask
- TONYMOLY INTENSE CARE SNAIL
- Walgreens Children GlycerinLaxative
- DR JOE LAB VITAMIN C SERUM
- Glycerol, Vetec(TM) reagent grade
- Leaders Mediu Nutrition Grain Pack
- Pearl ReWhite Transfusion DNA Kit
- Pink Sweet Potato Facial Cleansing
- Shine Control Sebum Regulating Gel
- WELLAGE Vita Red Injec-tion Mask
- YEJIMIIN Feminine Wash Jeju Herb
- Evereden Multi-purpose Healing Balm
- HY-B1659
- isLeaf Botanic Shield Shieldig Mist
- LANBELLE EGF DUO MOISTURIZER
- NATURALTH GOAT MILK MOISTURE
- NOBLESSE HOMME REFRESH TONER
- Perfect V Lifting Premium Eye Mask
- QUEEN79 NOBLE GOLD EYECREAM
- STR02073
- TAMHADA II Synergy Whitening Mask
- TONYMOLY My Sunny Watery Sun Gel
- WELLAGE Vital Nio Waterlock Toner
- 3CE LIQU ID EYE LINER BLACK
- 3CE LIQU ID EYE LINER BROWN
- Blister BalmProtective Lip Treatment
- Dr. A Anti Pollution Pollustop Ato
- Saphire ReHydro Transfusion DNA Kit
- SKINBUTAK GO YOUNG MAGICCREAM
- SKINBUTAK GOLD MAGIC CLEANSER
- SNAIL BEE HIGH CONTENT120mL
- TAMHADA II Synergy Moisturing Mask
- Tox21_111043
- Tox21_202077
- Tox21_300144
- Tracys Dog Personal Lubricant300mL
- WELLAGE Vital Nio Nutritional Rich
- WELLAGE WRINKLE REDUCED VIALS
- c0066
- DAG(31:3)
- Dr.NUELL Propolis Real 90 Ampoule
- Glycerol, BioXtra, >=99% (GC)
- Glycerol, ReagentPlus(R), >=99%
- MAG(17:1)
- MEDI HYDRO DP STEM C AMPOULE
- NSC759633
- Perfect V Lifting Premium Plus Mask
- QUEEN79 NOBLE GOLD AQUACREAM
- s2766
- STL199174
- C-MORE Glycerin Lubricant Eye Drops
- Fedora CONCENTRATING HYDRO SERUM
- Fedora CONCENTRATING HYDRO TONER
- OERBEUA HEATING PORE CRAY PACK
- Perfect V Lifting Premium Black Mask
- PLACEUTICA WIRE LIFT FACE NECK
- Premium natural BL hydrogel mask pack
- SKINBUTAK GO YOUNG MAGICLOTION
- The21 DAYs HAIR NUTRIENT SERUM
- AKOS000120102
- CHICA Y CHICO Killing Star Cleanser
- DANCHUN JADE oriental herb cosmetics
- DIAPIA 24K Gold SnailGel Eye Patch
- DIONA CELL ILLUMINATING BOOSTER
- DIONA CELL REGENERATION BOOSTER
- Dr Vita Clinic Gently Vita Exfoliator
- Hydra lifting Firming Nourishing Cream
- Hydra Lifting Firming Plus Serum 24H
- Leaders Mediu Clearing Strawberry Pack
- OERBEUA PURIFYING CLEANSER FOAM
- SISEUNDEUSI BLESS BATHANDSHAMPOO
- Tourmaline Relief Transfusion DNA Kit
- Truezyme Active Enzyme Scalp and Hair
- Vitalizing Energizing Nourishing Cream
- 1,2,3-TRIHYDROXYPROPAN-2-YL
- Amethyst ReElastic Transfusion DNA Kit
- AN ADC SP INTENSIVE WRINKLE EYE
- AN12 Secret Therapy Feminine Cleanser
- CS-6964
- DB09462
- DR. GLODERM TABRX MOISTURE MASK
- Glycerol, USP, 99.0-101.0%
- Hydra Lifting Firming Fresh Serum 24H
- NSC-759633
- REPIEL PERFECT FIT MASK FIRMING
- SB83762
- Stratuscare Glycerin Laxative Pediatric
- aewajin Derma Hydrocell 24k Gold Serum
- GLYCERIN; PROPANE-1,2,3-TRIOL
- Glycerol in water for HPLC verification
- Hyalucollagen Moisturizercombination skin
- Leaders Mediu Moisturizing Jasmine Pack
- Renovating Regenerating Nourishing Cream
- REPIEL PERFECT FIT MASK SOOTHING
- SELLA CLASSIC NATURAL GOLD SERUM
- BANACOS Cica-aid Centella Peptide toner
- CLENZIDERM THERAPEUTIC MOISTURIZER
- DIAPIA 24K Gold Snail FirmingGel Mask
- Glycerol, SAJ first grade, >=98.0%
- Hydra Lifting Firming Moisturizing Cream
- Lightening Brightening Moisturizing Serum
- NCGC00090950-01
- NCGC00090950-02
- NCGC00090950-04
- NCGC00090950-05
- NCGC00253975-01
- NCGC00259626-01
- REPIEL SMART FOOT MASK ULTRA RICH
- SISEUNDEUSI LUMINANT PACK CLEANSER
- WELLAGE BLACK AQUA ROLL MASK PACK
- WELLAGE Concentrated Illumination Expert
- BP-31039
- DR. GLODERM TIME TO MOISTURE MASK
- E422
- Ginsenoside Rg1 Rejuvenating Moisturizing
- Glycerin Liquid Laxative Enema(Kaisalilu)
- Glycerol, for molecular biology, >=99%
- Glycerol, JIS special grade, >=99.0%
- Glycerol, Vetec(TM) reagent grade, 99%
- Jena Cell VL Volume Lift Gravity Essence
- MG(17:1)
- NATURALTH GOAT MILK MOISTURE TONER
- Renovating Regenerating Moisturizing Cream
- REPIEL PERFECT FIT MASK LIGHTENING
- REPIEL TIMELESS AQUA SHOT BIO MASK
- REPIEL TIMELESS LINE SHOT BIO MASK
- REPIEL TIMELESS MELA SHOT BIO MASK
- SURIA PREMIUM JOJOBA FOAM CLEANSER
- Glycerin, meets USP testing specifications
- Lavietox B Project Re Set Firming Essence
- LEADERS MEDIU BRIGHTENING MILK PACK
- Mecell Rose Coenzyme Q10 Serum Mask Pack
- OSEQUE CYBER SHINE Oxygen Mask Cleanser
- Pororo Friends Smart Pore Guide Mask Pack
- SELLA CLASSIC NATURAL CLEANSING BAR
- SELLA PREMIUM NATURAL CLEANSING BAR
- 1ST001281
- Balmers Hanryeoncho Scalp Scooling mud pack
- Fedora CONCENTRATING HYDRO MOISTURIZER
- Hyalucollagen MoisturizerNormal and dry skin
- OASIS TearsPreservative-Free Lubricant Eye
- PLACEUTICA WIRE LIFT FACE FIT AMOULE
- Pororo Friends Tong Tong Firming Mask Pack
- REPIEL PERFECT FIT MASK MOISTURIZING
- TAMHADA II Synergy Anti-Wrinkle Face Mask
- G0316
- Glycerol, ultrapure, Spectrophotometric Grade
- IM SORRY FOR MY SKIN MASK-BRIGHTENING
- NS00004097
- REPIEL SMART HAND MASK MILKY MOISTURE
- REPIEL TIMELESS REDNESS SHOT BIO MASK
- By Pharmicell Lab Beaucell Dual Hydrogel Mask
- DOCTORS CHOICE Glycerin Lubricant Eye Drops
- EN300-19328
- Glycerol [Matrix for FABMS and liquid SIMS]
- Glycerol, ReagentPlus(R), >=99.0% (GC)
- Glycerol, spectrophotometric grade, >=99.5%
- IM SORRY FOR MY SKIN MASK-REVITALIZING
- INTENSE CARE GALACTOMYCES FIRST ESSENCE
- isLeaf Botanic Shield Deep Pore Cleasing Foam
- isLeaf Botanic Shield Facial Barrier GelCream
- Oasis Tears PFPreservative-Free Lubricant Eye
- QYO QYO Tangerine Bright Moist Foam Cleanser
- Age Defense Pro Prebiotic Technology Econature
- BK Cell 5DAYS OF SECRET MYSTIC CLEANSING
- C00116
- D00028
- D92249
- isLeaf Botanic Shield Pollutant Defending Mask
- PLACEUTICA WIRE LIFT FACE FIT 365 MASK
- Tonymoly Latte Art Cappuccino Cre am In Scrub
- TONYMOLY NATURALTH GOAT MILK PREMIUM EYE
- Vela Contour Neck and chin toning firming serum
- BK Cell 5DAYS OF SECRET AQUA MOISTURIZING
- CVS Pharmacy GLYCERIN SUPPOSITORIESLAXATIVE
- Dr. Althea Foaming Cleanser and Bubble O2 Mask
- MIZON ENJOY VITAL UP TIME Anti wrinkle Mask
- OASIS Tears PlusPreservative-Free Lubricant Eye
- A831186
- B BIND REJUVENATING NEOENDORPHIN MASK PACK
- DIAPIA ADL TURMERIC PEARL FOAMING CLEANSER
- Glycerol, tested according to Ph.Eur., anhydrous
- J9 ULTRA ADVANCED INTENSIVE RECOVERY SERUM
- Q132501
- The All Medicare V PLA Synergy Moisturizing Mask
- Biellee Pollen Whitening Wrinkle Time Repair Ampoule
- Oasis TEARS PF PLUSPreservative-Free Lubricant Eye
- PRO RADIANCE RENASCENT AN INFINITE ENERGIZER
- QUEENS ROSE ELYSEE CONCEN TRATE DUAL AMPOULE
- BK Cell 5DAYS OF SECRET PURITY WRINKLE LIFTING
- BRD-K73866522-001-02-6
- Glycerol-Gelatine, for mounting (histochemical slides)
- CVS Pharmacy Childrens GLYCERIN SUPPOSITORIESLAXATIVE
- F0001-1470
- LANBELLE WHITENING GELMASKPACK GRAPEFRUIT THERAPY
- Li om FERMENTATION FORM ELASTIC CARE SECRET SERUM
- 8DFDFCD7-1ED2-4373-845E-054F5AD00089
- InChI=1/C3H8O3/c4-1-3(6)2-5/h3-6H,1-2H
- MODLINA PEPTIDE SOOTHING ESSENTIAL HYDROGEL MASK PACK
- WELLAGE Double Lift Mask Pack Moisturizing Hydrogel Mask
- Glycerin, United States Pharmacopeia (USP) Reference Standard
- La Roche Posay Laboratoire Dermatologique Cicaplast Defense B5
- PRO RADIANCE RENASCENT AN INFINITE ENERGIZER MOISTURIZER
- Glycerin based Gentle and Effective Laxative Adult Constipation Relief
- Glycerin, Pharmaceutical Secondary Standard; Certified Reference Material
- Glycerol, BioUltra, for molecular biology, anhydrous, >=99.5% (GC)
- Glycerol, p.a., ACS reagent, reag. ISO, reag. Ph. Eur., 98.0-101.0%
- Glycerol, puriss. p.a., ACS reagent, anhydrous, dist., >=99.5% (GC)
- La Roche Posay Laboratoire Dermatologique Cicaplast Defense B5 Skin Protectant
- 26403-55-4
- Glycerol, BioReagent, suitable for cell culture, suitable for insect cell culture, suitable for electrophoresis, >=99% (GC)
- Glycerol, puriss., meets analytical specification of Ph. Eur., BP, USP, FCC, E422, anhydrous, 99.0-101.0% (alkalimetric)
- Schoenflies notation
- Boiling point
- Chemical diffusion
- Composition
- Compressibility
- Corrosion
- Critical point
- Crystal structure
- Density
- Diamagnetic susceptibility
- Dielectric constant
- Diffusion
- Diffusive flux
- Electron conductivity
- Formula unit
- Fusion temperature
- Heat capacity
- Heat of sublimation
- Magnetic susceptibility
- Melting temperature
- Molecular structure
- Optical coefficient
- Phase diagram
- Phase equilibrium
- Phase transition
- Point group
- Refractive index
- Self-diffusion
- Shock waves
- Sound absorption
- Sound propagation
- Sound velocity
- Space group
- Structure formula
- Surface tension
- Thermal expansion coefficient
- Transition enthalpy
- Unit cell
- Unit cell parameter
- Vapor pressure
- Vapor-liquid equilibrium
- Viscosity
205.0 1
117.0 0.96
103.0 0.86
133.0 0.60
218.0 0.32
61.0 99.99
43.0 80.03
44.0 47.21
31.0 45.01
29.0 25.52
61.0 1
43.0 0.80
44.0 0.47
31.0 0.45
29.0 0.26
57.389 100
57.513 93.31
75.065 15.09
75.563 10.63
45 999
44 343
91 106
90 48
61 999
43 800
44 472
31 450
29 255
Polyglycerin-3 (monomer of)
- Tobacco Leaf (part of)
Polyglyceryl-3 Diisostearate (monomer of)
Polyglyceryl-4 caprate (monomer of)
- Polyglyceryl-10 myristate (monomer of)
Polyglyceryl-3 laurate (monomer of)
- Polyglyceryl-4 isostearate (monomer of)
- Polyglyceryl-6 distearate (monomer of)
Polyglyceryl-10 stearate (monomer of)
- Polyglyceryl-10 oleate (monomer of)
- Polyglyceryl-6 isostearate (monomer of)
- Polyglyceryl-6 ricinoleate (monomer of)
- Polyglycerin-6 (monomer of)
- Polyglyceryl-10 caprylate (monomer of)
Polyglyceryl-10 laurate (monomer of)
- Polyglyceryl-6 behenate (monomer of)
- Polyglyceryl-4 laurate (monomer of)
- Polyglycerin-10 (monomer of)
- Polyglyceryl-6 Dioleate (monomer of)
- Polyglycerin-4 (monomer of)
- Harufilcon A (monomer of)
- Polyglyceryl-10 decaoleate (monomer of)
- Polyglyceryl-10 isostearate (monomer of)
- Polyglyceryl-10 hexaoleate (monomer of)
- Polyglyceryl-10 pentaoleate (monomer of)
- Polyglyceryl-10 pentastearate (monomer of)
- Polyglyceryl-6 stearate (monomer of)
Polyglyceryl-3 ricinoleate (monomer of)
- Glycerin; phenol (component of)
- Glycerin; lidocaine (component of)
- Glycerin; povidone (component of)
- Polyglyceryl-10 dipalmitate (monomer of)
- Polyglyceryl-10 tetraoleate (monomer of)
- Polyglyceryl-10 dioleate (monomer of)
- Polyglyceryl-10 caprate (monomer of)
- Polyglyceryl-4 oleate (monomer of)
- Polyglyceryl-4 stearate (monomer of)
- Polyglyceryl-6 polyricinoleate (monomer of)
- Adenosine; Glycerin (component of)
- Arbutin; glycerin (component of)
- Glycerin; iodine (component of)
- Allantoin; Glycerin (component of)
- Bronopol; glycerin (component of)
- Biotin; glycerin (component of)
- Glycerin; propylene glycol (component of)
- Aloe; glycerin (component of)
- Glycerin; kaolin (component of)
- Glycerin; silver (component of)
- Glycerin; menthol (component of)
- Polyglyceryl-5 trioleate (monomer of)
- Polyglycerin-20 (monomer of)
- Polyglyceryl-4 polyricinoleate (monomer of)
- Polyglycerin-40 (monomer of)
Trimethylpentanediol/adipic acid/glycerin crosspolymer (25000 MPA.S) (monomer of)
- Dextran 70; Glycerin; Hypromelloses (component of)
- Carboxymethylcellulose Sodium; Glycerin (component of)
- Glycerin; Isopropyl Alcohol (component of)
- Amber; glycerin; laureth-10 (component of)
- Glycerin; lanolin; lidocaine; zinc oxide (component of)
- Adenosine; arbutin; glycerin (component of)
- Glycerin; mineral oil (component of)
- Dimethicone; Glycerin (component of)
- Glycerin; sodium borate (component of)
- Glycerin; phenylephrine hydrochloride (component of)
- Glycerin; hydrogen peroxide (component of)
- Glycerin; lidocaine; phenylephrine hydrochloride (component of)
- Glycerin; silver cation (component of)
- Glycerin; Niacinamide (component of)
- Glycerin; polysorbate 80 (component of)
- Glycerin; kaolin; menthol; zinc oxide (component of)
- Aloe vera leaf; edetate disodium; glycerin; urea (component of)
- Glycerin; naphazoline hydrochloride; zinc sulfate (component of)
- Glycerin; glycolic acid (component of)
- Glycerin; methylpropanediol (component of)
- Polyglyceryl-10 tetralinoleate (monomer of)
- Polyglyceryl-4 palmitoleate (monomer of)
- Diisostearoyl polyglyceryl-3 dimer dilinoleate (monomer of)
- Polyglyceryl-10 polyricinoleate (monomer of)
- Polyglyceryl-4 linoleate (monomer of)
- Polyglyceryl-4 succinate (monomer of)
- Polyglyceryl-5 polyricinoleate (monomer of)
- Polyglyceryl-4 palmitate (monomer of)
- Allantoin; glycerin; mineral oil (component of)
- Glycerin; hypromellose, unspecified (component of)
- Adenosine; glycerin; niacinamide (component of)
- Adenosine; caviar, unspecified; glycerin (component of)
- Glycerin; hyaluronate sodium; pork collagen (component of)
- Adansonia digitata fruit; glycerin (component of)
- Glycerin; hypromelloses; polyethylene glycol 400 (component of)
- Dimethicone; glycerin; panthenol (component of)
- Glycerin; lactic acid; salicylic acid (component of)
- Glycerin; lidocaine; witch hazel; zinc oxide (component of)
- Allantoin; Glycerin; Niacinamide (component of)
- Carboxymethylcellulose sodium; glycerin; polysorbate 80 (component of)
- Glycerin; Thermus Thermophilus Lysate (component of)
- Glycerin; methyl perfluoroisobutyl ether (component of)
- Polyglycerol polyricinoleic acid (monomer of)
- Aloe vera leaf; glycerin; propylene glycol; tocopherol (component of)
- Citric acid monohydrate; glycerin; pinus radiata bark (component of)
- Glycerin; lidocaine; petrolatum; phenylephrine hydrochloride (component of)
- Fibroblast growth factor-1; glycerin; hyaluronate sodium (component of)
- Glycerin; nelumbo nucifera flower; niacinamide; purslane (component of)
- Butylene glycol; glycerin; inonotus obliquus fruiting body (component of)
- Glycerin; lidocaine; phenylephrine hydrochloride; white petrolatum (component of)
- Glycerin; silicon dioxide; sodium monofluorophosphate (component of)
- Glyceryl acrylate/acrylic acid copolymer (300000 CP AT 2%) (monomer of)
- Glycerin; Petrolatum; Phenylephrine Hydrochloride; Pramoxine Hydrochloride (component of)
- Citric acid monohydrate; glycerin; menthol; pinus radiata bark (component of)
- Alcohol; aluminum chloride; formaldehyde; glycerin; methyl alcohol (component of)
- Polyglyceryl-3 polydimethylsiloxyethyl dimethicone (4000 mpa.S) (monomer of)
- Amentoflavone; asian ginseng; biotin; ginger; glycerin; pinus palustris leaf (component of)
- Glycerin; hypromellose, unspecified; polyethylene glycol 400; tetrahydrozoline hydrochloride; zinc sulfate (component of)
- Alcohol; ferulic acid; fragrance 13576; glycerin; lecithin, soybean; malondialdehyde; peg-8 caprylic/capric glycerides; polyoxyl 35 castor oil; water (component of)
- Benzoic acid; butylene glycol; dehydroacetic acid; edetic acid; fragrance 13576; glycerin; phenoxyethanol; polysorbate 20; propanediol; proteoglycan 4; water; xanthan gum (component of)
- BENZOIC ACID; BENZYL ALCOHOL; DEHYDROACETIC ACID; EDETIC ACID; FRAGRANCE 13576; GLYCERIN; HYDROXYCITRONELLAL; ISOMETHYL-alpha-IONONE; PEG-8 CAPRYLIC/CAPRIC GLYCERIDES; PHENOXYETHANOL; PROPANEDIOL; SORBIC ACID; WATER (component of)
- Amanita muscaria fruiting body; ambergris; barium carbonate; colchicum autumnale bulb; convallaria majalis; ginkgo; glycerin; nutmeg; riboflavin; scutellaria lateriflora whole; semecarpus anacardium juice; sus scrofa cerebrum (component of)
- Acmella oleracea flowering top; apis mellifera; atropa belladonna; baptisia tinctoria root; calcium sulfide; clove; echinacea angustifolia whole; glycerin; goldenseal; lachesis muta venom; mercurius solubilis; myrrh; phytolacca americana root; sage; thymus serpyllum whole (component of)
- Bos taurus pituitary gland, posterior; epinephrine; glycerin; glycyrrhiza glabra; lactic acid, DL-; lactic acid, L-; lactose, unspecified form; magnesium sulfate heptahydrate; nickel; pancrelipase; phosphoric acid; potassium carbonate; saccharin; selenium; silver nitrate; sucrose; syzygium cumini seed; thyroid, unspecified; zinc (component of)
- beta-CITRONELLOL, (R)-; ALOE VERA LEAF; BAICALIN; BENZYL ALCOHOL; CETOSTEARYL ALCOHOL; DECYL OLEATE; DICAPRYLYL ETHER; EUGENOL; FRAGRANCE 13576; GLYCERIN; GLYCERYL MONOSTEARATE; HYDROXYCITRONELLAL; JUJUBE SEED; LECITHIN, SOYBEAN; LEUZEA CARTHAMOIDES ROOT; LIMONENE, (+)-; LINALOOL, (+/-)-; PROPANEDIOL; SALICYLIC ACID; SHEA BUTTER; SORBIC ACID; TOCOPHEROL; WATER; XANTHAN GUM (component of)
- beta-CITRONELLOL, (R)-; ALCOHOL; BUTYLATED HYDROXYTOLUENE; C12-20 ACID PEG-8 ESTER; CETEARYL ETHYLHEXANOATE; ETHYLHEXYLGLYCERIN; FRAGRANCE 13576; GLYCERIN; GLYCERYL MONOSTEARATE; GRAPE SEED OIL; HYDROXYACETOPHENONE; ISOMETHYL-alpha-IONONE; LINALOOL, (+/-)-; PALMITIC ACID; PERLITE; PHENOXYETHANOL; PROPYLENE GLYCOL; STARCH, RICE; STEARIC ACID; TITANIUM DIOXIDE; TROLAMINE; WATER (component of)
- 1,2-Hexanediol; allantoin; aloe vera leaf; butylated hydroxytoluene; C12-20 acid peg-8 ester; caprylyl glycol; carbomer homopolymer, unspecified type; cetearyl ethylhexanoate; cetyl alcohol; decyl oleate; dimethicone; fragrance 13576; glycerin; glyceryl monostearate; glyceryl stearate SE; imidurea; medium-chain triglycerides; octinoxate; oxybenzone; propylene glycol; tocopherol; trolamine; tropolone; water (component of)
- Avena sativa flowering top; berberis vulgaris root bark; bos taurus testicle; bufo bufo cutaneous gland; chaste tree fruit; cinchona officinalis bark; egg phospholipids; epimedium grandiflorum top; ferric chloride hexahydrate; glycerin; iodine; phosphoric acid; phosphorus; saw palmetto; selenium; sepia officinalis juice; sodium chloride; somatropin; sus scrofa ovary; turnera diffusa leafy twig; zinc valerate dihydrate (component of)
- 1,2-Hexanediol; allantoin; aloe vera leaf; argan oil; butylated hydroxytoluene; butyrospermum parkii (shea) butter unsaponifiables; C12-20 acid peg-8 ester; caprylyl glycol; carbomer homopolymer, unspecified type; decyl oleate; fragrance 13576; glycerin; grape seed oil; hexamethylene diisocyanate/trimethylol hexyllactone crosspolymer; imidurea; medium-chain triglycerides; propylene glycol; silicon dioxide; tocopherol; trolamine; tropolone; water (component of)
- alpha-HEXYLCINNAMALDEHYDE; beta-CITRONELLOL, (R)-; AVOBENZONE; BENZOIC ACID; BUTYLATED HYDROXYTOLUENE; CENTAUREA CYANUS FLOWER; CETOSTEARYL ALCOHOL; CETYL ALCOHOL; COCO-GLYCERIDES; DEHYDROACETIC ACID; FRAGRANCE 13576; GLYCERIN; HYDROLYSED MARINE COLLAGEN (ENZYMATIC; 2000 MW); HYDROLYZED BOVINE ELASTIN (BASE; 1000 MW); ISOMETHYL-alpha-IONONE; LIMONENE, (+)-; MEDIUM-CHAIN TRIGLYCERIDES; OCTINOXATE; PHENOXYETHANOL; POTASSIUM PHOSPHATE, UNSPECIFIED FORM; VIOLA ODORATA; WATER (component of)
- Acetic acid; arsenic trioxide; aspirin; benzoic acid; benzyl alcohol; boric acid; chlorine; corticotropin; cortisone acetate; estrone; eugenol; glycerin; insulin human; isopropyl palmitate; kerosene; lactic acid, DL-; lead; lithium carbonate; nitric acid; nitroglycerin; petrolatum; phenylbutazone; phosphoric acid; phosphorus; phytolacca americana root; potassium sorbate; resorcinol; salicylic acid; sorbitol; stearyl alcohol; strychnos nux-vomica seed; taraxacum officinale; thyroid, porcine; xylitol (component of)
- Acetic acid; arsenic trioxide; aspirin; benzoic acid; benzyl alcohol; boric acid; chlorine; corticotropin; cortisone acetate; estrone; eugenol; glycerin; insulin pork; isopropyl palmitate; kerosene; lactic acid, DL-; lead; lithium carbonate; nitric acid; nitroglycerin; petrolatum; phenylbutazone; phosphoric acid; phosphorus; phytolacca americana root; potassium sorbate; resorcinol; salicylic acid; sorbitol; stearyl alcohol; strychnos nux-vomica seed; sus scrofa thyroid; taraxacum officinale; xylitol (component of)
- Ascorbic acid; ascorbyl palmitate; avobenzone; bemotrizinol; butylated hydroxytoluene; butylene glycol; C20-22 alcohols; carbomer copolymer type A (allyl pentaerythritol crosslinked); carbomer homopolymer, unspecified type; chlorphenesin; dimethicone; fragrance 13576; glycerin; linoleic acid; linolenic acid; octinoxate; octisalate; octocrylene; phenoxyethanol; poly(methyl methacrylate; 450000 MW); polyethylene glycol 400; propylene glycol; silicon dioxide; titanium dioxide; tocopherol; tromethamine; water (component of)
- alpha-TOCOPHEROL; ANHYDROUS CITRIC ACID; ASCORBIC ACID; ASCORBYL PALMITATE; AVOBENZONE; BEMOTRIZINOL; BUTYLATED HYDROXYTOLUENE; BUTYLENE GLYCOL; C20-22 ALCOHOLS; CARBOMER COPOLYMER TYPE A (ALLYL PENTAERYTHRITOL CROSSLINKED); CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE; CHLORPHENESIN; DIMETHICONE; FRAGRANCE 13576; GLYCERIN; LINOLEIC ACID; LINOLENIC ACID; OCTINOXATE; OCTISALATE; OCTOCRYLENE; PHENOXYETHANOL; POLY(METHYL METHACRYLATE; 450000 MW); POLYETHYLENE GLYCOL 400; PROPYLENE GLYCOL; SILICON DIOXIDE; TITANIUM DIOXIDE; TROMETHAMINE; WATER (component of)
- 2-Propenoic acid, polymer with oxirane and 1,2,3-propanetriol (annotation moved to)
- Glycerine (mist) (annotation moved to)
- Glycerol, ethoxylated, esters with Acrylic acid (annotation moved to)
Flavouring Agent ->
FLAVOURING_AGENTFood Additives -> CARRIER_SOLVENT; EMULSIFIER; HUMECTANT; THICKENER;
- Adipose Tissue
- Bladder
- Brain
- Epidermis
- Kidney
- Liver
- Neuron
- Pancreas
- Placenta
- Prostate
- Skeletal Muscle
- Spleen
- Testis
- Thyroid Gland
- Extracellular
- Mitochondria
Pulp and Paper Processing [Category: Industry]
Textiles (Printing, Dyeing, or Finishing) [Category: Industry]
 Green circle - The chemical has been verified to be of low concern
Green circle - The chemical has been verified to be of low concernFlavouring Agent -> -> JECFA Functional Classes
FLAVOURING_AGENTFood Additives -> CARRIER_SOLVENT; EMULSIFIER; HUMECTANT; THICKENER; -> JECFA Functional Classes
- Plasticizer
- Lubricating agent
- Processing aids, not otherwise listed
- Catalyst
- Surfactant (surface active agent)
- Process regulators
- Humectant
- Processing aids not otherwise specified
- Abrasives
- Dye
- Not Known or Reasonably Ascertainable
- Intermediate
- Solvent
- Sealant (barrier)
- Intermediates
- Adhesives and sealant chemicals
- Viscosity modifiers
- Surface modifier
- Laboratory chemicals
- Other (specify)
- Lubricants and lubricant additives
- Adhesives and sealant chemicals
- Not Known or Reasonably Ascertainable
- Sealant (barrier)
- Solvent
- Plasticizers
- Pigment
- Viscosity modifiers
- Solvents (for cleaning or degreasing)
- Other (specify)
- Adhesion/cohesion promoter
- Flavoring and nutrient
- Lubricating agent
- Surfactant (surface active agent)
- Agricultural chemicals (non-pesticidal)
- Surface active agents
- Plasticizer
- Soil amendments (fertilizers)
- Dye
- Other
- Binder
Information on 2656 consumer products that contain Glycerin in the following categories is provided:
• Auto Products
• Commercial / Institutional
• Hobby/Craft
• Home Maintenance
• Home Office
• Inside the Home
• Landscaping/Yard
• Personal Care
• Pesticides
• Pet Care
Glycerin (Glycerol) preparations: (AHFS, 2011)
Table: Glycerin (Glycerol) preparations: (AHFS, 2011)
2019: 1,000,000,000 - <5,000,000,000 lb
2018: 1,000,000,000 - <5,000,000,000 lb
2017: 1,000,000,000 - <5,000,000,000 lb
2016: 1,000,000,000 - <5,000,000,000 lb
Glycerol production in the United States:
Table: Glycerol Production (100% glycerol basis) in the United States, Metric Tons
- Oil and Gas Drilling, Extraction, and Support activities
- Construction
- All Other Chemical Product and Preparation Manufacturing
- Printing and Related Support Activities
- Non-metallic Mineral Product Manufacturing (includes clay, glass, cement, concrete, lime, gypsum, and other non-metallic mineral product manufacturing)
- Not Known or Reasonably Ascertainable
- Plastics Material and Resin Manufacturing
- Soap, Cleaning Compound, and Toilet Preparation Manufacturing
- Utilities
- Rubber Product Manufacturing
- Fabricated Metal Product Manufacturing
- Plastics Product Manufacturing
- Printing Ink Manufacturing
- Pharmaceutical and Medicine Manufacturing
- All Other Basic Organic Chemical Manufacturing
- Pesticide, Fertilizer, and Other Agricultural Chemical Manufacturing
- Other (requires additional information)
- Food, beverage, and tobacco product manufacturing
- Petroleum Lubricating Oil and Grease Manufacturing
- Wholesale and Retail Trade
- Miscellaneous Manufacturing
Not Classified
Reported as not meeting GHS hazard criteria by 5500 of 5595 companies (only 1.7% companies provided GHS information). For more detailed information, please visit ECHA C&L website.
Aggregated GHS information provided per 5595 reports by companies from 16 notifications to the ECHA C&L Inventory.
Reported as not meeting GHS hazard criteria per 5500 of 5595 reports by companies. For more detailed information, please visit ECHA C&L website.
There are 13 notifications provided by 95 of 5595 reports by companies with hazard statement code(s).
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.

Chemical: Glycerol

EYES: First check the victim for contact lenses and remove if present. Flush victim's eyes with water or normal saline solution for 20 to 30 minutes while simultaneously calling a hospital or poison control center. Do not put any ointments, oils, or medication in the victim's eyes without specific instructions from a physician. IMMEDIATELY transport the victim after flushing eyes to a hospital even if no symptoms (such as redness or irritation) develop.
SKIN: IMMEDIATELY flood affected skin with water while removing and isolating all contaminated clothing. Gently wash all affected skin areas thoroughly with soap and water. If symptoms such as redness or irritation develop, IMMEDIATELY call a physician and be prepared to transport the victim to a hospital for treatment.
INHALATION: IMMEDIATELY leave the contaminated area; take deep breaths of fresh air. If symptoms (such as wheezing, coughing, shortness of breath, or burning in the mouth, throat, or chest) develop, call a physician and be prepared to transport the victim to a hospital. Provide proper respiratory protection to rescuers entering an unknown atmosphere. Whenever possible, Self-Contained Breathing Apparatus (SCBA) should be used; if not available, use a level of protection greater than or equal to that advised under Protective Clothing.
INGESTION: DO NOT INDUCE VOMITING. If the victim is conscious and not convulsing, give 1 or 2 glasses of water to dilute the chemical and IMMEDIATELY call a hospital or poison control center. Be prepared to transport the victim to a hospital if advised by a physician. If the victim is convulsing or unconscious, do not give anything by mouth, ensure that the victim's airway is open and lay the victim on his/her side with the head lower than the body. DO NOT INDUCE VOMITING. IMMEDIATELY transport the victim to a hospital. (NTP, 1992)
(See general first aid procedures)
Eye: Irrigate immediately - If this chemical contacts the eyes, immediately wash (irrigate) the eyes with large amounts of water, occasionally lifting the lower and upper lids. Get medical attention immediately.
Skin: Water wash - If this chemical contacts the skin, wash the contaminated skin with water.
Breathing: Fresh air
N.D.
See: IDLH INDEX
Excerpt from NIOSH Pocket Guide for Glycerin (mist):
Skin: No recommendation is made specifying the need for personal protective equipment for the body.
Eyes: No recommendation is made specifying the need for eye protection.
Wash skin: No recommendation is made specifying the need for washing the substance from the skin (either immediately or at the end of the work shift).
Remove: No recommendation is made specifying the need for removing clothing that becomes wet or contaminated.
Change: No recommendation is made specifying the need for the worker to change clothing after the workshift. (NIOSH, 2024)
(See personal protection and sanitation codes)
Skin: No recommendation
Eyes: No recommendation
Wash skin: No recommendation
Remove: No recommendation
Change: No recommendation
IMAP assessments - 1,2,3-Propanetriol: Human health tier I assessment
IMAP assessments - 1,2,3-Propanetriol: Environment tier I assessment
PubMed: 15026783, 6299616, 9007327, 20110216
MetaGene: Metabolic & Genetic Information Center (MIC: http://www.metagene.de)
World Health Organisation Department of Noncommunicable Disease Surveillance (1999). "Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications"
Rosa Va ́zquez-Fresno et al. An NMR metabolomics approach reveals a combined-biomarkers model in a wine interventional trial with validation in free-living individuals of the PREDIMED study. Metabolomics (2015) 11:797- 806: https://link.springer.com/content/pdf/10.1007/s11306-014-0735-x.pdf
The Merck Manual, 17th ed. Mark H. Beers, MD, Robert Berkow, MD, eds. Whitehouse Station, NJ: Merck Research Labs, 1999.
Silke Matysik, Caroline Ivanne Le Roy, Gerhard Liebisch, Sandrine Paule Claus. Metabolomics of fecal samples: A practical consideration. Trends in Food Science & Technology. Vol. 57, Part B, Nov. 2016, p.244-255: http://www.sciencedirect.com/science/article/pii/S0924224416301984
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=PEDCQBHIVMGVHV-UHFFFAOYSA-N
- Australian Industrial Chemicals Introduction Scheme (AICIS)1,2,3-Propanetriolhttps://services.industrialchemicals.gov.au/search-assessments/1,2,3-Propanetriolhttps://services.industrialchemicals.gov.au/search-inventory/
- CAMEO ChemicalsLICENSECAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data.https://cameochemicals.noaa.gov/help/reference/terms_and_conditions.htm?d_f=falseCAMEO Chemical Reactivity Classificationhttps://cameochemicals.noaa.gov/browse/react
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/Poly[oxy(2-hydroxy-1,3-propanediyl)], α-hydro-ω-hydroxy-https://commonchemistry.cas.org/detail?cas_rn=26403-55-4
- ChemIDplusChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DrugBankLICENSECreative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode)https://www.drugbank.ca/legal/terms_of_use
- DTP/NCILICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuse
- EPA Chemical Data Reporting (CDR)LICENSEThe U.S. Government retains a nonexclusive, royalty-free license to publish or reproduce these documents, or allow others to do so, for U.S. Government purposes. These documents may be freely distributed and used for non-commercial, scientific and educational purposes.https://www.epa.gov/web-policies-and-procedures/epa-disclaimers#copyright1,2,3-Propanetriolhttps://www.epa.gov/chemical-data-reporting
- EPA Chemicals under the TSCA1,2,3-Propanetriolhttps://www.epa.gov/chemicals-under-tscaEPA TSCA Classificationhttps://www.epa.gov/tsca-inventory
- EPA DSSTox1,2,3-Trihydroxypropan-2-ylhttps://comptox.epa.gov/dashboard/DTXSID10991197CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-notice1,2,3-Propanetriolhttps://echa.europa.eu/substance-information/-/substanceinfo/100.120.095Glycerol (EC: 200-289-5)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/2536
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Hazardous Substances Data Bank (HSDB)
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citingHMDB0000131_cms_1007https://hmdb.ca/metabolites/HMDB0000131#spectra
- ILO-WHO International Chemical Safety Cards (ICSCs)
- International Fragrance Association (IFRA)LICENSE(c) The International Fragrance Association, 2007-2021. All rights reserved.https://ifrafragrance.org/links/copyright
- New Zealand Environmental Protection Authority (EPA)LICENSEThis work is licensed under the Creative Commons Attribution-ShareAlike 4.0 International licence.https://www.epa.govt.nz/about-this-site/general-copyright-statement/
- Risk Assessment Information System (RAIS)LICENSEThis work has been sponsored by the U.S. Department of Energy (DOE), Office of Environmental Management, Oak Ridge Operations (ORO) Office through a joint collaboration between United Cleanup Oak Ridge LLC (UCOR), Oak Ridge National Laboratory (ORNL), and The University of Tennessee, Ecology and Evolutionary Biology, The Institute for Environmental Modeling (TIEM). All rights reserved.https://rais.ornl.gov/
- The National Institute for Occupational Safety and Health (NIOSH)LICENSEThe information provided using CDC Web site is only intended to be general summary information to the public. It is not intended to take the place of either the written law or regulations.https://www.cdc.gov/Other/disclaimer.htmlGlycerin (mist)https://www.cdc.gov/niosh/npg/npgd0302.html
- EU Food Improvement Agents
- Haz-Map, Information on Hazardous Chemicals and Occupational DiseasesLICENSECopyright (c) 2022 Haz-Map(R). All rights reserved. Unless otherwise indicated, all materials from Haz-Map are copyrighted by Haz-Map(R). No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://haz-map.com/AboutGlycerinhttps://haz-map.com/Agents/501
- ChEBI
- E. coli Metabolome Database (ECMDB)
- FDA Pharm ClassesLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingFDA Pharmacological Classificationhttps://www.fda.gov/ForIndustry/DataStandards/StructuredProductLabeling/ucm162549.htm
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/LOTUS Treehttps://lotus.naturalproducts.net/
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- Open TargetsLICENSEDatasets generated by the Open Targets Platform are freely available for download.https://platform-docs.opentargets.org/licence
- Toxin and Toxin Target Database (T3DB)LICENSET3DB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (T3DB) and the original publication.http://www.t3db.ca/downloads
- Yeast Metabolome Database (YMDB)
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- Comparative Toxicogenomics Database (CTD)LICENSEIt is to be used only for research and educational purposes. Any reproduction or use for commercial purpose is prohibited without the prior express written permission of NC State University.http://ctdbase.org/about/legal.jsp
- Consumer Product Information Database (CPID)LICENSECopyright (c) 2024 DeLima Associates. All rights reserved. Unless otherwise indicated, all materials from CPID are copyrighted by DeLima Associates. No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://www.whatsinproducts.com/contents/view/1/6Consumer Products Category Classificationhttps://www.whatsinproducts.com/
- Cosmetic Ingredient Review (CIR)
- EPA Chemical and Products Database (CPDat)EPA CPDat Classificationhttps://www.epa.gov/chemical-research/chemical-and-products-database-cpdat
- NIST Synthetic Polymer MALDI Recipes Databaseglycerolhttps://maldi.nist.gov/
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/GlycerolNORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- DailyMed
- Drugs@FDALICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- ECI Group, LCSB, University of Luxembourgglycerol
- KNApSAcK Species-Metabolite Database
- Natural Product Activity and Species Source (NPASS)
- West Coast Metabolomics Center-UC DavisGlycerol
- EPA Safer ChoiceEPA Safer Chemical Ingredients Classificationhttps://www.epa.gov/saferchoice
- FDA Orange BookLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Joint FAO/WHO Expert Committee on Food Additives (JECFA)LICENSEPermission from WHO is not required for the use of WHO materials issued under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Intergovernmental Organization (CC BY-NC-SA 3.0 IGO) licence.https://www.who.int/about/policies/publishing/copyright
- USGS Columbia Environmental Research CenterLICENSEhttps://www.usgs.gov/foia
- EU Clinical Trials Register
- National Drug Code (NDC) DirectoryLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- FDA Substances Added to FoodLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Flavor and Extract Manufacturers Association (FEMA)
- FooDBLICENSEFooDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (FooDB) and the original publication.https://foodb.ca/about
- IUPAC Digitized pKa Dataset1,2,3-Propanetriolhttps://github.com/IUPAC/Dissociation-Constants
- NMRShiftDB
- MassBank Europe
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-law
- SpectraBase1,2,3-PROPANETRIOLhttps://spectrabase.com/spectrum/AOOOWATuMHFGlycerin, U.S.P.https://spectrabase.com/spectrum/CDFTLWCCCyP1,2,3-Propanetriolhttps://spectrabase.com/spectrum/2lL121sHJQE1,2,3-Propanetriolhttps://spectrabase.com/spectrum/6iqmsRYAfqv
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlCompounds with biological roleshttp://www.genome.jp/kegg-bin/get_htext?br08001.kegTherapeutic category of drugs in Japanhttp://www.genome.jp/kegg-bin/get_htext?br08301.kegAnatomical Therapeutic Chemical (ATC) classificationhttp://www.genome.jp/kegg-bin/get_htext?br08303.kegDrugs listed in the Japanese Pharmacopoeiahttp://www.genome.jp/kegg-bin/get_htext?br08311.kegRisk category of Japanese OTC drugshttp://www.genome.jp/kegg-bin/get_htext?br08312.kegClassification of Japanese OTC drugshttp://www.genome.jp/kegg-bin/get_htext?br08313.kegAnimal drugs in Japanhttp://www.genome.jp/kegg-bin/get_htext?br08331.keg
- MarkerDBLICENSEThis work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.https://markerdb.ca/Glycerolhttps://markerdb.ca/chemicals/82
- Metabolomics Workbench
- Nature Chemical Biology
- Nature Chemistry
- NIPH Clinical Trials Search of Japan
- NLM RxNorm TerminologyLICENSEThe RxNorm Terminology is created by the National Library of Medicine (NLM) and is in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from NLM. Credit to the U.S. National Library of Medicine as the source is appreciated but not required. The full RxNorm dataset requires a free license.https://www.nlm.nih.gov/research/umls/rxnorm/docs/termsofservice.html
- Protein Data Bank in Europe (PDBe)
- RCSB Protein Data Bank (RCSB PDB)LICENSEData files contained in the PDB archive (ftp://ftp.wwpdb.org) are free of all copyright restrictions and made fully and freely available for both non-commercial and commercial use. Users of the data should attribute the original authors of that structural data.https://www.rcsb.org/pages/policies
- Rhea - Annotated Reactions DatabaseLICENSERhea has chosen to apply the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/). This means that you are free to copy, distribute, display and make commercial use of the database in all legislations, provided you credit (cite) Rhea.https://www.rhea-db.org/help/license-disclaimer
- Springer Nature
- SpringerMaterialspropane-1,2,3-triolhttps://materials.springer.com/substanceprofile/docs/smsid_vikvruyigmwspwir
- The Cambridge Structural Database
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- WHO Anatomical Therapeutic Chemical (ATC) ClassificationLICENSEUse of all or parts of the material requires reference to the WHO Collaborating Centre for Drug Statistics Methodology. Copying and distribution for commercial purposes is not allowed. Changing or manipulating the material is not allowed.https://www.whocc.no/copyright_disclaimer/
- Wikidata
- Wikipedia
- Wiley
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlCryoprotective Agentshttps://www.ncbi.nlm.nih.gov/mesh/68003451
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- Glycan Naming and Subsumption Ontology (GNOme)GNOme
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403029528https://pubchem.ncbi.nlm.nih.gov/substance/403029528
- NCBI