Oncovin
- C46H56N4O10.H2O4S
- C46H58N4O14S
- Vincristine sulfate
- 2068-78-2
- Vincristine sulfate salt
- Oncovin
- Vincristine sulphate
- Create:2005-06-29
- Modify:2024-12-06
 Vincristine Sulfate (annotation moved to).
Vincristine Sulfate (annotation moved to).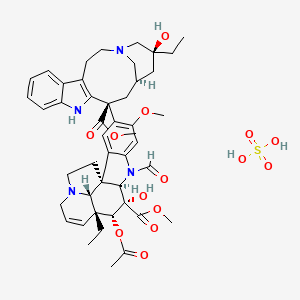
- cellcristin
- Citomid
- Farmistin
- Leurocristine
- Oncovin
- Oncovine
- Onkocristin
- PFS, Vincasar
- Sulfate, Vincristine
- Vincasar
- Vincasar PFS
- Vincristin Bristol
- Vincristin medac
- Vincristine
- Vincristine Sulfate
- Vincrisul
- Vintec
- Vincristine sulfate
- 2068-78-2
- Vincristine sulfate salt
- Oncovin
- Vincristine sulphate
- Vincristine (sulfate)
- Vincrex
- Kyocristine
- Vincrisul
- Onkovin
- MLS000863277
- T5IRO3534A
- Marqibo Kit
- VCR sulfate
- Vincaleukoblastine, 22-oxo-, sulfate (1:1) (salt)
- SMR000058540
- Vincristine Sulfate PFS
- Lilly 37231
- Leurocristine sulfate (1:1) (salt)
- Vincasar PFS
- Vincristinsulfat
- MLS000069706
- Leurocristine, sulfate (1:1) (salt)
- Vincristinsulfat [German]
- UNII-T5IRO3534A
- VINCRISTINE SULFATE [IARC]
- VINCRISTINE SULFATE [MART.]
- CCRIS 2583
- VINCRISTINE SULFATE [USP-RS]
- VINCRISTINE SULFATE [EP MONOGRAPH]
- Vincristine sulfate; Methyl (3aR,4R,5S,5aR,10bR,13aR)-4-(acetyloxy)-3a-ethyl-9-[(5S,7R,9S)-5-ethyl-5-hydroxy-9-(methoxycarbonyl)-1,4,5,6,7,8,9,10-octahydro-2H-3,7-methanoazacycloundecino[5,4-b]indol-9-yl]-6-formyl-5-hydroxy-8-methoxy-3a,4,5,5a,6,11,12,13a-octahydro-1H-indolizino[8,1-cd]carbazole-5-carboxylate sulfate
- EINECS 218-190-0
- VINCRISTINE SULFATE [USP MONOGRAPH]
- MFCD00084729
- Vincristine sulfate [USAN:USP:JAN]
- Vincristine Sulfate Liposomes Injection
- Opera_ID_836
- AI3-52944
- Leurocristine sulfate salt
- Vincristine sulfate (assay)
- MLS001148250
- MLS001401434
- CHEMBL385978
- VINCRISTINE SULFATE [MI]
- VINCRISTINE SULFATE [JAN]
- AQTQHPDCURKLKT-PNYVAJAMSA-N
- HMS2052D03
- HMS2234B06
- VINCRISTINE SULFATE [USAN]
- VINCRISTINE SULFATE [VANDF]
- HY-N0488
- VINCRISTINE SULFATE [WHO-DD]
- VINCRISTINE SULFATE [WHO-IP]
- 22-Oxovincaleukoblastine sulfate salt
- AKOS030497863
- CCG-101152
- CS-1778
- NC00402
- VCR . sulfate;Leukocristine . sulfate
- VINCRISTINE SULFATE [ORANGE BOOK]
- AS-12168
- VINCRISTINI SULFAS [WHO-IP LATIN]
- VCR
- Q-201925
- Q27095645
- Vincristine sulfate, meets USP testing specifications
- Vincristine sulfate salt, 95.0-105.0% (HPLC), powder
- 22-OXOVINCALEUKOBLASTINE SULFATE (1:1) (SALT) [WHO-IP]
- Vincristine sulfate, European Pharmacopoeia (EP) Reference Standard
- Vincristine sulfate, United States Pharmacopeia (USP) Reference Standard
- Vincristine sulfate (assay), United States Pharmacopeia (USP) Reference Standard
 Vincristine Sulfate (annotation moved to)
Vincristine Sulfate (annotation moved to)Vincristine sulfate is approved to treat adults and children with:
• Acute leukemia.
Vincristine sulfate is sometimes used to treat adults and children with other types of cancer, including:
• Hodgkin lymphoma.
• Neuroblastoma.
• Non-Hodgkin lymphoma (NHL).
• Rhabdomyosarcoma.
• Wilms tumor.
Vincristine sulfate is also being studied in the treatment of other types of cancer.


H300 (91.3%): Fatal if swallowed [Danger Acute toxicity, oral]
H341 (89.1%): Suspected of causing genetic defects [Warning Germ cell mutagenicity]
H361 (91.3%): Suspected of damaging fertility or the unborn child [Warning Reproductive toxicity]
P203, P264, P270, P280, P301+P316, P318, P321, P330, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 46 reports by companies from 8 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Acute Tox. 2 (91.3%)
Muta. 2 (89.1%)
Repr. 2 (91.3%)
SYMPTOMS: The following symptoms of exposure have occurred during intravenous use. Symptoms of exposure include bone marrow depression, peripheral neuropathy, colicky abdominal pain, constipation and alopecia. Other symptoms include neuromuscular and neurological disturbances, gastrointestinal upset and leucopenia. It has caused impaired walking, convulsions, hypertension, inappropriate antidiuretic hormone (ADH) secretion, jaw pain, paresthesias in the fingers and toes, sensory impairment, headache, loss of deep tendon reflexes, parotid gland pain, optic atrophy and blindness. It has also caused acute uric acid nephropathy, myocardial infarction, ocular toxicity (ptosis, other ocular muscle paresis, and 5th and 7th nerve involvement); laryngeal nerve paralysis causing hoarseness or cough, depression of the Achilles tendon reflex, muscle pain, weakness, motor weakness, quadriparesis, numbness and tingling of the fingers and toes, malaise, depression, psychoses, neuromyopathy, peripheral neuritis, adynamic ileus, and permanent central nervous system damage. Exposure may cause paresthesia, foot drop, ataxia, athetosis, thrombocytosis and coma. Exposure may also cause granulocytopenia, thrombocytopenia, hypoplasia of all elements of bone marrow, nausea, vomiting, anorexia, neuritic pain and motor difficulties. Other effects may include cranial nerve neuropathy including optic nerve neuropathy and injury to the retina; cataracts, facial paralysis, diplopia and corneal hypesthesia. It may cause anemia, polyuria, dysuria, fever, tingling and numbness of the extremities, abdominal obstruction, ischemic cardiac toxicity and syndrome of hyponatremia. It may also cause diarrhea, sensory loss, slapping gait, muscle wasting, generalized sensorimotor dysfunction, paralytic ileus, abdominal cramps, rash, oral ulceration, intestinal necrosis and/or perforation, urinary retention due to bladder atony cranial nerve manifestations including isolated paresis and/or paralysis of muscles controlled by cranial motor nerves; pharyngeal pain, bone pain, back pain, limb pain, myalgias, renal or adrenal disease, hypotension, dehydration, azotemia and clinical edema. Effects on the autonomic nervous system have been reported. It may cause irritation of the skin, and congenital malformation in the fetus.
ACUTE/CHRONIC HAZARDS: This compound is highly toxic and may be fatal if inhaled, swallowed or absorbed through the skin. It may cause irritation of the skin. When heated to decomposition it emits very toxic fumes of carbon monoxide, carbon dioxide, nitrogen oxides and sulfur oxides. (NTP, 1992)
EYES: First check the victim for contact lenses and remove if present. Flush victim's eyes with water or normal saline solution for 20 to 30 minutes while simultaneously calling a hospital or poison control center. Do not put any ointments, oils, or medication in the victim's eyes without specific instructions from a physician. IMMEDIATELY transport the victim after flushing eyes to a hospital even if no symptoms (such as redness or irritation) develop.
SKIN: IMMEDIATELY flood affected skin with water while removing and isolating all contaminated clothing. Gently wash all affected skin areas thoroughly with soap and water. If symptoms such as redness or irritation develop, IMMEDIATELY call a physician and be prepared to transport the victim to a hospital for treatment.
INHALATION: IMMEDIATELY leave the contaminated area; take deep breaths of fresh air. IMMEDIATELY call a physician and be prepared to transport the victim to a hospital even if no symptoms (such as wheezing, coughing, shortness of breath, or burning in the mouth, throat, or chest) develop. Provide proper respiratory protection to rescuers entering an unknown atmosphere. Whenever possible, Self-Contained Breathing Apparatus (SCBA) should be used; if not available, use a level of protection greater than or equal to that advised under Protective Clothing.
INGESTION: DO NOT INDUCE VOMITING. Strychnine is an exceptionally toxic poison but inducing vomiting may cause a seizure. IMMEDIATELY call a hospital or poison control center and locate activated charcoal, egg whites, or milk in case the medical advisor recommends administering one of them. If advice from a physician is not readily available and the victim is conscious and not convulsing, give the victim a glass of activated charcoal slurry in water or, if this is not available, a glass of milk, or beaten egg whites and IMMEDIATELY transport victim to a hospital. If the victim is convulsing or unconscious, do not give anything by mouth, assure that the victim's airway is open and lay the victim on his/her side with the head lower than the body. DO NOT INDUCE VOMITING. IMMEDIATELY transport the victim to a hospital. (NTP, 1992)
Excerpt from ERG Guide 151 [Substances - Toxic (Non-Combustible)]:
IMMEDIATE PRECAUTIONARY MEASURE: Isolate spill or leak area in all directions for at least 50 meters (150 feet) for liquids and at least 25 meters (75 feet) for solids.
SPILL: Increase the immediate precautionary measure distance, in the downwind direction, as necessary.
FIRE: If tank, rail tank car or highway tank is involved in a fire, ISOLATE for 800 meters (1/2 mile) in all directions; also, consider initial evacuation for 800 meters (1/2 mile) in all directions. (ERG, 2024)
SMALL SPILLS AND LEAKAGE: If you spill this chemical, you should dampen the solid spill material with water, then transfer the dampened material to a suitable container. Use absorbent paper dampened with water to pick up any remaining material. Seal your contaminated clothing and the absorbent paper in a vapor-tight plastic bag for eventual disposal. Wash all contaminated surfaces with a soap and water solution. Do not reenter the contaminated area until the Safety Officer (or other responsible person) has verified that the area has been properly cleaned.
STORAGE PRECAUTIONS: You should protect this chemical from exposure to light. Keep the container tightly closed under an inert atmosphere, and store under refrigerated temperatures. (NTP, 1992)
Alcohols and Polyols
Amides and Imides
Esters, Sulfate Esters, Phosphate Esters, Thiophosphate Esters, and Borate Esters
Salts, Acidic
Hazard Traits - Developmental Toxicity
Authoritative List - Prop 65
Report - regardless of intended function of ingredient in the product
IMAP assessments - Vincaleukoblastine, 22-oxo-, sulfate (1:1) (salt): Human health tier I assessment
IMAP assessments - Vincaleukoblastine, 22-oxo-, sulfate (1:1) (salt): Environment tier I assessment
Volume 26: (1981) Some Antineoplastic and Immunosuppressive Agents
Volume Sup 7: Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs Volumes 1 to 42, 1987; 440 pages; ISBN 92-832-1411-0 (out of print)
◉ Summary of Use during Lactation
Most sources consider breastfeeding to be contraindicated during maternal antineoplastic drug therapy. It is probably impractical to resume breastfeeding after vincristine therapy because of the drug's long half-life. Chemotherapy may adversely affect the normal microbiome and chemical makeup of breastmilk.
◉ Effects in Breastfed Infants
In a 4-month-old, neutropenia was probably caused by cyclophosphamide in a mother 9 days after the last of 6 weekly doses of 800 mg cyclophosphamide intravenously, 2 mg vincristine intravenously and daily doses of 30 mg of prednisolone orally. Neutropenia persisted at least 12 days and was accompanied by a brief episode of diarrhea. The contribution of vincristine to the neutropenia cannot be determined.
A woman was diagnosed with B-cell lymphoma at 27 weeks of pregnancy. Labor was induced at 34 4/7 weeks and treatment was begun with a standard regimen of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone in unspecified doses on a 21-day cycle, starting on day 2 postpartum. She pumped and discarded her milk and fed her infant donor milk for the first 10 days of each cycle and then breastfed her infant for the remaining 10 days before the next treatment cycle. The 10-day period of breastfeeding abstinence was determined by using about 3 half-lives of vincristine. After completion of 4 cycles of chemotherapy, her infant was reportedly healthy and developing without any complications.
◉ Effects on Lactation and Breastmilk
Relevant published information was not found as of the revision date.
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=AQTQHPDCURKLKT-PNYVAJAMSA-N
- Australian Industrial Chemicals Introduction Scheme (AICIS)Vincaleukoblastine, 22-oxo-, sulfate (1:1) (salt)https://services.industrialchemicals.gov.au/search-assessments/
- CAMEO ChemicalsLICENSECAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data.https://cameochemicals.noaa.gov/help/reference/terms_and_conditions.htm?d_f=falseVINCRISTINE SULFATEhttps://cameochemicals.noaa.gov/chemical/21221CAMEO Chemical Reactivity Classificationhttps://cameochemicals.noaa.gov/browse/react
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/Vincristine sulfatehttps://commonchemistry.cas.org/detail?cas_rn=2068-78-2
- ChemIDplusVincristine sulfate [USAN:USP:JAN]https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0002068782ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeVincristine sulphatehttps://echa.europa.eu/substance-information/-/substanceinfo/100.016.537Vincristine sulphate (EC: 218-190-0)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/4346
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingVINCRISTINE SULFATEhttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/T5IRO3534A
- California Office of Environmental Health Hazard Assessment (OEHHA)Vincristine Sulfatehttps://oehha.ca.gov/proposition-65/chemicals/vincristine-sulfate
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- California Safe Cosmetics Program (CSCP) Product DatabaseVincristine sulfatehttps://www.cdph.ca.gov/Programs/CCDPHP/DEODC/OHB/CSCP/Pages/About-CSCP.aspx
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.html
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- DailyMed
- Drug Gene Interaction database (DGIdb)LICENSEThe data used in DGIdb is all open access and where possible made available as raw data dumps in the downloads section.http://www.dgidb.org/downloadsVINCRISTINE SULFATEhttps://www.dgidb.org/drugs/rxcui:11203
- Drugs and Lactation Database (LactMed)
- Drugs@FDALICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingVINCRISTINE SULFATEhttps://www.accessdata.fda.gov/scripts/cder/daf/
- EU Clinical Trials Register
- FDA Orange BookLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- International Agency for Research on Cancer (IARC)LICENSEMaterials made available by IARC/WHO enjoy copyright protection under the Berne Convention for the Protection of Literature and Artistic Works, under other international conventions, and under national laws on copyright and neighbouring rights. IARC exercises copyright over its Materials to make sure that they are used in accordance with the Agency's principles. All rights are reserved.https://publications.iarc.fr/Terms-Of-UseVincristine sulfatehttps://monographs.iarc.who.int/list-of-classificationsIARC Classificationhttps://www.iarc.fr/
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.html
- National Drug Code (NDC) DirectoryLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- NCI Cancer DrugsVincristine Sulfatehttps://www.cancer.gov/about-cancer/treatment/drugs/vincristinesulfate
- NIPH Clinical Trials Search of Japan
- NLM RxNorm TerminologyLICENSEThe RxNorm Terminology is created by the National Library of Medicine (NLM) and is in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from NLM. Credit to the U.S. National Library of Medicine as the source is appreciated but not required. The full RxNorm dataset requires a free license.https://www.nlm.nih.gov/research/umls/rxnorm/docs/termsofservice.htmlvincristine sulfatehttps://rxnav.nlm.nih.gov/id/rxnorm/11203
- SpectraBaseVincristine sulfate salthttps://spectrabase.com/spectrum/LQBXcY4n8BP
- Springer Nature
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlVincristinehttps://www.ncbi.nlm.nih.gov/mesh/68014750Tubulin Modulatorshttps://www.ncbi.nlm.nih.gov/mesh/68050257Antineoplastic Agents, Phytogenichttps://www.ncbi.nlm.nih.gov/mesh/68000972
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 390168376https://pubchem.ncbi.nlm.nih.gov/substance/390168376
 CID 5388993 (Citomid)
CID 5388993 (Citomid) CID 1118 (Sulfuric Acid)
CID 1118 (Sulfuric Acid)