Leu-Asn
PubChem CID
7016078
Molecular Formula
Synonyms
- H-Leu-Asn-OH
- Leu-Asn
- 14608-81-2
- L-leucyl-L-asparagine
- L-Leu-L-Asn
Molecular Weight
245.28 g/mol
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Dates
- Create:2006-07-29
- Modify:2024-12-07
Description
Leu-Asn is a dipeptide composed of L-leucine and L-asparagine joined by a peptide linkage. It has a role as a metabolite. It is functionally related to a L-leucine and a L-asparagine.
Chemical Structure Depiction
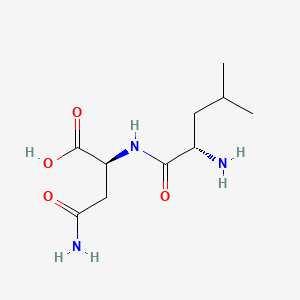
SVG Image

IUPAC Condensed
H-Leu-Asn-OH
Sequence
LN
PLN
H-LN-OH
HELM
PEPTIDE1{L.N}$$$$
IUPAC
L-leucyl-L-asparagine
(2S)-4-amino-2-[[(2S)-2-amino-4-methylpentanoyl]amino]-4-oxobutanoic acid
Computed by Lexichem TK 2.7.0 (PubChem release 2021.10.14)
InChI=1S/C10H19N3O4/c1-5(2)3-6(11)9(15)13-7(10(16)17)4-8(12)14/h5-7H,3-4,11H2,1-2H3,(H2,12,14)(H,13,15)(H,16,17)/t6-,7-/m0/s1
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
MLTRLIITQPXHBJ-BQBZGAKWSA-N
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
CC(C)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)N
Computed by OEChem 2.3.0 (PubChem release 2021.10.14)
C10H19N3O4
Computed by PubChem 2.2 (PubChem release 2021.10.14)
- H-Leu-Asn-OH
- Leu-Asn
- 14608-81-2
- L-leucyl-L-asparagine
- L-Leu-L-Asn
- CHEBI:73529
- leucylasparagine
- (2S)-4-amino-2-[[(2S)-2-amino-4-methylpentanoyl]amino]-4-oxobutanoic acid
- (2S)-4-amino-2-[[(2S)-2-azaniumyl-4-methylpentanoyl]amino]-4-oxobutanoate
- (S)-4-Amino-2-((S)-2-amino-4-methylpentanamido)-4-oxobutanoic acid
- Leucyl-asparagin
- (2S)-4-amino-2-(((2S)-2-amino-4-methylpentanoyl)amino)-4-oxobutanoic acid
- (2S)-4-amino-2-(((2S)-2-azaniumyl-4-methylpentanoyl)amino)-4-oxobutanoate
- asparagine, leucyl-
- MFCD00057937
- CHEMBL1222158
- HY-P4374
- LN
- DA-64194
- CS-0653765
- Q27140611
- L-N
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
245.28 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
XLogP3
Property Value
-4.5
Reference
Computed by XLogP3 3.0 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Donor Count
Property Value
4
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Acceptor Count
Property Value
5
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Rotatable Bond Count
Property Value
7
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Exact Mass
Property Value
245.13755610 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Monoisotopic Mass
Property Value
245.13755610 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Topological Polar Surface Area
Property Value
136Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Heavy Atom Count
Property Value
17
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
304
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
2
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
MoNA ID
MS Category
Experimental
MS Type
Other
MS Level
MS2
Precursor Type
[M+H]+
Precursor m/z
246.1448326
Ionization Mode
positive
Retention Time
1.982866666667
Top 5 Peaks
133.06406322502212 0.35
86.09718222502211 0.28
87.0532452250221 0.07
116.03940522502211 0.05
229.1161887641098 0.05
MoNA ID
MS Category
Experimental
MS Type
Other
MS Level
MS2
Precursor Type
[M-H]-
Precursor m/z
244.1302796
Ionization Mode
negative
Retention Time
1.9748
Top 5 Peaks
41.99819842476348 0.34
96.00866642476348 0.16
129.10301042476348 0.12
114.01728042476347 0.06
112.07596042476348 0.06
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Parent, Connectivity Count
Same Parent, Exact Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=MLTRLIITQPXHBJ-BQBZGAKWSA-N
- ChEBI
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- IUPAC Digitized pKa Datasetasparagine, leucyl-https://github.com/IUPAC/Dissociation-Constants
- Japan Chemical Substance Dictionary (Nikkaji)
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- Metabolomics Workbench
- Springer Nature
- Wikidata
- PubChem
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/LOTUS Treehttps://lotus.naturalproducts.net/
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 387545344https://pubchem.ncbi.nlm.nih.gov/substance/387545344
CONTENTS



